h
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Confidential draft submitted to the Securities and Exchange Commission on August 27, 2018. This draft registration statement has not been filed publicly with the Securities and Exchange Commission and all information contained herein remains confidential.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10
GENERAL FORM FOR REGISTRATION OF SECURITIES
PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934
_____________________________
|
DIAMEDICA THERAPEUTICS INC. (Exact name of registrant as specified in its charter) |
||
|
Canada (State or other jurisdiction of incorporation or organization) |
33-1224644 (I.R.S. Employer Identification No.) |
|
|
2 Carlson Parkway, Suite 260 Minneapolis, Minnesota 55447 (Address of principal executive offices) (Zip Code) |
||
|
(763) 496-5454 (Registrant’s telephone number, including area code)
Rick Pauls President and Chief Executive Officer DiaMedica Therapeutics Inc. 2 Carlson Parkway, Suite 260 Minneapolis, Minnesota 55447 (763) 496-5454 (Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to: Amy E. Culbert, Esq. Brett R. Hanson, Esq. Fox Rothschild LLP 222 South Ninth Street, Suite 2000 Minneapolis, MN 55402 (612) 607-7000 |
||
_________________
Securities to be registered under Section 12(b) of the Act:
|
Title of each class to be so registered |
Name of each exchange on which each class is to be registered |
|
|
Voting Common Shares |
The Nasdaq Stock Market LLC |
|
|
Voting Common Share Purchase Rights |
The Nasdaq Stock Market LLC |
Securities to be registered under Section 12(g) of the Act:
None
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
|
Accelerated filer ☐ |
||
|
Non-accelerated filer ☐ |
(Do not check if a smaller reporting company) |
|
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
INFORMATION REQUIRED IN REGISTRATION STATEMENT
TABLE OF CONTENTS
Page
|
EXPLANATORY NOTE |
ii |
|
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS |
iii |
|
|
ITEM 1. |
BUSINESS. |
1 |
|
ITEM 1.A. |
RISK FACTORS |
16 |
|
ITEM 2. |
FINANCIAL INFORMATION. |
33 |
|
ITEM 3. |
PROPERTIES. |
39 |
|
ITEM 4. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT. |
40 |
|
ITEM 5. |
DIRECTORS AND EXECUTIVE OFFICERS. |
42 |
|
ITEM 6. |
EXECUTIVE COMPENSATION. |
45 |
|
ITEM 7. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
51 |
|
ITEM 8. |
LEGAL PROCEEDINGS. |
52 |
|
ITEM 9. |
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS. |
52 |
|
ITEM 10. |
RECENT SALES OF UNREGISTERED SECURITIES. |
55 |
|
ITEM 11. |
DESCRIPTION OF REGISTRANT’S SECURITIES TO BE REGISTERED. |
57 |
|
ITEM 12. |
INDEMNIFICATION OF DIRECTORS AND OFFICERS. |
60 |
|
ITEM 13. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
61 |
|
ITEM 14. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
61 |
|
ITEM 15. |
FINANCIAL STATEMENTS AND EXHIBITS. |
61 |
|
SIGNATURES |
63 |
|
|
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS |
F-1 |
|
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
EXPLANATORY NOTE
DiaMedica Therapeutics Inc. is filing this registration statement on Form 10 pursuant to Section 12(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), because we are seeking to list our voting common shares, no par value (“common shares”), on the Nasdaq Capital Market (“Nasdaq”). We are a “smaller reporting company,” as defined by Rule 12b-2 of the Exchange Act, and an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”).
Our common shares currently trade in Canada on the TSX Venture Exchange under the trading symbol “DMA” and in the United States on the OTCQB Market under the trading symbol “DMCAF.” We have applied to list our common shares on Nasdaq under the trading symbol “DMAC.”
In order to meet the minimum bid price listing standard required by Nasdaq in connection with our Nasdaq listing application, we intend to effect a share consolidation, or reverse stock split, of our common shares, prior to the effective date of this registration statement. At our Annual General and Special Meeting of Shareholders held on December 21, 2017, our shareholders approved a reverse split at a rate of up to one-for-thirty (1:30), with the actual rate to be determined by our Board of Directors in its sole discretion; provided, that the split must be effected no later than December 21, 2018. Our Board of Directors has not yet determined the actual reverse split ratio or timing of the split. The selection of the reverse split ratio will be based primarily on the trading price of our common shares at the time and anticipated stability at that level. The reverse stock split will not affect the number of our authorized common shares. We must obtain approval of the TSX Venture Exchange prior to effecting the reverse stock split.
Unless we indicate otherwise, the information in this registration statement does not reflect the pro forma impact of the reverse stock split. We intend to update the information in this registration statement prior to its effective date to reflect the pro forma impact of the reverse stock split. If we effect the reverse stock split, then, without further action on the part of our shareholders, the outstanding common shares held by shareholders of record as of the effective date of the reverse stock split will be converted into a fewer number of common shares based on the reverse stock split ratio. For example, if the Board of Directors approves a reverse split ratio of one-for-ten (1:10), then a holder of 10,000 common shares would hold 1,000 common shares immediately following the reverse stock split. No fractional shares will be issued in connection with the reverse stock split. In the event that a shareholder would otherwise be entitled to receive a fractional share in connection with the reverse stock split, the number of common shares to be received by such shareholder will be rounded up or down to the nearest whole common share.
We do not currently file periodic reports with the United States Securities and Exchange Commission (the “SEC”). When this registration statement becomes effective, we will be required to file annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements and other information with the SEC pursuant to the Exchange Act. You may read and copy this information, for a copying fee, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information on its Public Reference Room. Our SEC filings will also be available to the public at the website maintained by the SEC at http://www.sec.gov.
Our internet website address is http://www.diamedica.com. Information contained on our website does not constitute part of this registration statement. When this registration statement becomes effective, we will make available on our website, through a link to the SEC’s website, electronic copies of the documents we file with the SEC.
As used in this registration statement, unless otherwise specified or the context otherwise requires, “DiaMedica,” “we,” “our,” “us” and the “Company” refer to DiaMedica Therapeutics Inc. and our subsidiaries, and references to “CAD$” and “Canadian dollars” are to the lawful currency of Canada and references to “$” and “US$” and “U.S. dollars” are to the lawful currency of the United States. All dollar amounts herein are in U.S. dollars, unless otherwise indicated.
DiaMedica Therapeutics Inc. and our logo are our trademarks. This registration statement also includes trademarks, tradenames and service marks that are the property of us and of other organizations. Solely for convenience, our trademarks and tradenames referred to in this registration statement appear without any “™” or “®” symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of any applicable licensor, to these trademarks and tradenames.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
SPECIAL NOTE REGARDING INDUSTRY DATA
Unless otherwise indicated, information contained in this registration statement concerning DiaMedica, our business, our product candidates, our industry and our general expectations concerning our product candidates and industry are based on management estimates. Such estimates are derived from publicly available information released by third party sources, as well as data from our internal research, and reflect assumptions made by us based on such data and our knowledge of the industry, which we believe to be reasonable.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this registration statement that are not descriptions of historical facts are forward-looking statements that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would” or the negative of these terms or other comparable terminology. Factors that could cause actual results to differ materially from those currently anticipated include those set forth under Item 1.A. “Risk Factors” including, in particular, risks relating to:
|
● |
the results of research and development activities; |
|
● |
uncertainties relating to preclinical and clinical testing, financing and strategic agreements and relationships; |
|
● |
the early stage of our products under development; |
|
● |
our need for substantial additional funds; |
|
● |
government regulation; |
|
● |
our ability to obtain and maintain regulatory approval of our lead product candidate, DM199, and any of our other current or future product candidates; |
|
● |
our ability to retain key scientific or management personnel; |
|
● |
patent and intellectual property matters; |
|
● |
dependence on third parties; and |
|
● |
competition. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in the section Item 1.A. “Risk Factors.” Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this registration statement may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this registration statement to conform these statements to actual results or to changes in our expectations.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 1. |
BUSINESS. |
Overview
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant (synthetic) proteins. Our goal is to use our patented and licensed technologies to establish our company as a leader in the development and commercialization of novel recombinant proteins to treat neurological and kidney diseases. Our primary focus is on acute ischemic stroke (“AIS”) and chronic kidney disease (“CKD”). We plan to advance our lead drug candidate, DM199, through clinical trials, as appropriate, to create shareholder value by establishing its clinical and commercial potential as a therapy for AIS and CKD.
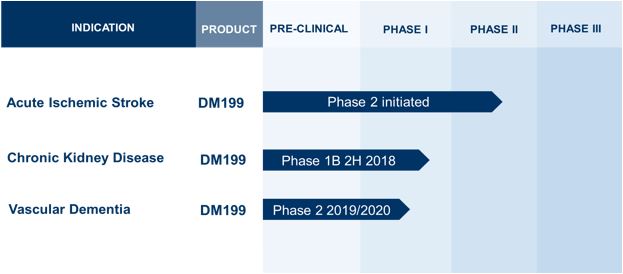
DM199 is a recombinant form of human tissue kallikrein-1 (“KLK1”). KLK1 is a serine protease (protein) produced in the pancreas, kidneys and salivary glands, which plays a critical role in the regulation of local blood flow and vasodilation (the widening of blood vessels which decreases blood pressure) in the body, as well as an important role in inflammation and oxidative stress (an imbalance between potentially damaging reactive oxygen species, or free radicals, and antioxidants in your body). We believe DM199 has the potential to treat a variety of diseases where healthy functioning requires sufficient activity of KLK1 and its system, the kallikrein-kinin system (“KKS”). The primary focus for our DM199 program development is on AIS and CKD; however, we also intend to pursue advancement in the vascular dementia market. We believe that our current Phase 1 clinical data will support the design and implementation of a Phase 2 study in vascular dementia. We have drafted a protocol synopsis for a Phase 2 study in vascular dementia that we intend to initiate when we are sufficiently capitalized. The current status of our product candidates in preclinical and clinical development is as follows:

KLK1 is involved in multiple biochemical processes. The most well-characterized activity of KLK1 is its enzymatic cleavage of low molecular weight kininogen (“LMWK”) to produce bradykinin (“BK”)-like peptides, collectively known as kinins, which activate BK receptors (BK1R, BK2R). Activation of BK receptors by kinins sets in motion metabolic pathways that can improve blood flow (through vasodilation), dampen inflammation, and protect tissues and end-organs from ischemic damage. Scientific literature, including publications in Circulation Research, Immunopharmacology and Kidney International, suggests that lower endogenous KLK1 levels in patients are associated with diseases related to vascular disorders, such as kidney diseases, stroke and hypertension. We believe DM199 could replenish endogenous KLK1 to properly activate the BK system that protects the kidney and brain from damage. By providing this additional supply of KLK1, DM199 treatment could improve blood flow to damaged end-organs, such as kidneys and brain, supporting the structural integrity and normal functioning. The current treatment options for these disorders cannot be used in all patients and are associated with serious side effects. We believe DM199 may provide significant benefits over the current standards of care by offering potentially fewer side effects and a therapeutic treatment option to a greater number of patients.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We are a corporation organized under the laws of Canada. Our company was initially incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of continuance dated January 21, 2000. Our articles of continuance were amended on February 26, 2001 to change our corporate name to DiaMedica Inc. and further amended on December 28, 2016 to change our corporate name to DiaMedica Therapeutics Inc. Our common shares currently trade in Canada on the TSX Venture Exchange under the trading symbol “DMA” and in the United States on the OTCQB Market under the trading symbol “DMCAF.” We have applied to list our common shares on Nasdaq under the trading symbol “DMAC.”
Our Strategy
Our goal is to become a leader in the discovery, development, and commercialization of recombinant proteins for the treatment of severe and life-threatening diseases. We seek to identify and select, for development and partnership, recombinant proteins with novel mechanisms that have biological properties with broad applicability. Once we have selected a class of recombinant proteins, we apply their biological properties to clinical settings with unmet needs, and we evaluate opportunities based on estimated development timelines and costs, regulatory pathway, and commercial opportunities. After identifying suitable molecules for clinical development, we intend to mitigate development risk by maintaining a diversified and broad clinical pipeline, rapidly analyzing data to determine the potential of each program and entering into development collaborations with industry-leading companies. Currently our strategy includes the following key components:
|
● |
DM199 for AIS - complete our ongoing Phase 2 study |
|
● |
DM199 for CKD - advance Phase 1b and Phase 2 studies |
|
● |
Leverage our technologies to expand our development pipeline |
|
● |
Use our expertise to identify and manufacture novel recombinant proteins |
Targeted Indications and Markets for DM199
Acute Ischemic Stroke
Stroke is characterized by the rapidly developing loss of brain function due to disturbance in the blood. As a result, the affected area of the brain becomes inactive and eventually dies. Strokes can be classified into two major categories: AIS and hemorrhagic stroke. AIS is characterized by interruption of the blood supply by a blood clot (ischemia), while a hemorrhagic stroke results from rupture, or bleeding, of a blood vessel or an abnormal vascular structure. According to the U.S. Center for Disease Control and Prevention, or CDC, about 87% of strokes are ischemic in nature with the remainder classified as hemorrhagic. According to the CDC, worldwide, stroke is an important cause of adult disability and the second leading cause of death in developed countries. Risk factors for stroke include advanced age, hypertension (high blood pressure), previous stroke or transient ischemic attack (“TIA”), diabetes, high cholesterol, cigarette smoking and atrial fibrillation. According to the World Health Organization, each year approximately 15 million people worldwide suffer a stroke, of which 5.5 million will die and 5.0 million will be permanently disabled. According to the CDC:
|
● |
Every year in the United States, approximately 795,000 people experience a new or recurrent stroke each year (ischemic or hemorrhagic). Approximately 610,000 of these are first events and 185,000 are recurrent stroke events. |
|
● |
Stroke caused approximately one of every 20 deaths in the United States. On average, someone in the United States has a stroke every 40 seconds, and someone dies from a stroke every four minutes. |
|
● |
Stroke costs the United States $34 billion annually, including the cost of health care services, medications and lost productivity. |
At the site of a blood flow blockage in the brain, there exist two major ischemic zones - the core ischemic zone with nearly complete loss of blood flow, and the surrounding ischemic penumbra having partially reduced blood flow. Within minutes, the significant lack of blood flow in the core (i.e. glucose and oxygen deprivation) rapidly depletes energy stores and triggers the loss of ion gradients, ultimately leading to neuronal cell death. The ischemic penumbra zone, however, may remain viable for several hours via collateral arteries that branch from the main occluded artery in the core zone. Unfortunately, the penumbra is at great risk of delayed tissue damage due to inflammation and cell death, or apoptosis.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
As time goes on, a lack of blood flow in the ischemic zone (infarct) leads to fluid buildup (edema) and swelling which creates intracranial pressure. This pressure on the brain leads to tissue compression resulting in additional ischemia. Additional events in AIS include vascular damage to the blood vessel lining or endothelium, loss of structural integrity of brain tissue and blood vessels, and inflammation. A stroke can lead to permanent damage with memory loss, speech problems, reading and comprehension difficulties, physical disabilities, and emotional/behavioral problems. The long-term costs of stroke are substantial, with many patients requiring extended hospitalization, extended physical therapy or rehabilitation, and/or long-term institutional or family care. However, provided the extended window of viability in the penumbra, next generation stroke therapies are being developed to protect valuable brain tissue during the hours to a week after a stroke.
We believe that stroke represents an area of significant unmet medical need, and a KLK1 treatment (such as DM199) could provide a significant patient benefit with its proposed therapeutic window of up to 24 hours after the first sign of symptoms. Currently, the only pharmacological intervention for AIS is the use of tissue plasminogen activator (“tPA”) which must be given within 4.5 hours of symptom onset. Mechanical thrombectomy, in which the clot is removed using catheter-based tools, is also available to some patients. Despite the availability of these treatments, many patients are not eligible due to the location of the clot, the elapsed time after the stroke occurred, or safety considerations. Thus, we believe DM199 offers significant advantages over the current treatment options and fills an unmet need for patients who cannot receive tPA. In fact, KLK1 in China (Kailikang®) is widely used for the treatment of AIS, making therapy available to hundreds of thousands of patients who currently have no options. Kailikang® is a human urine-extracted KLKl protein. We believe that the proprietary DM199 protein could result in an improved efficacy with optimized pharmacokinetics (drug level exposure) and avoid the side effects of risk of endotoxins, impurities and antibody formation in comparison to Kailikang® that is isolated from human urine. We also believe that DM199 addresses potential supply constraints that makes Kailikang® difficult and expensive to produce given the limited source of human urine. We believe these factors make the recombinant protein DM199 a product candidate that is better positioned for regulatory approval worldwide than a urine-derived protein since we believe it can meet the rigorous required manufacturing standards.
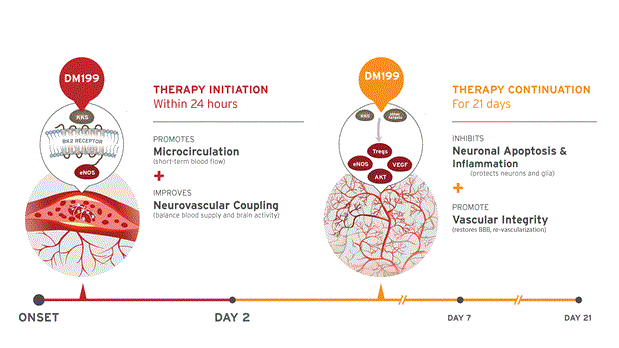
DM199 Acute Ischemic Stroke: Proposed Mechanism
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Chronic Kidney Disease
CKD is characterized by a progressive decline in overall kidney function as measured by glomerular filtration rate (“GFR”) (a test used to check how well the kidneys are filtering excess fluid and waste products out of your blood), and albuminuria (the amount of albumin protein excreted in your urine). When GFR gets too low, patients develop end stage renal disease (“ESRD”) and require dialysis or a kidney transplant to survive. Among multiple underlying causes, CKD often begins with an increase in blood glucose, which leads to the thickening of the glomerular membrane, known as fibrosis. As the kidney function becomes impaired, GFR decreases and abnormal amounts of protein are released into the urine collecting tubules of the kidney through damaged capillary pores. Additionally, increased blood glucose leads to increased blood pressure, reactive oxygen species, advanced glycation end product formation (harmful compounds that are formed when protein or fat combine with sugar in the bloodstream) and inflammation. As this continues, structural components of the kidney (the nephron) begin to collapse, resulting in cell ischemia and cell death. As the renal damage continues, a progressive thickening of the basement membrane is seen along with continued pathological changes in the cell and inflammation. Early stages of CKD are characterized as microalbuminuria (small amounts of protein leak into the urine). Late stages are characterized as macroalbuminuria (large amount of protein in the urine). The rate of decline depends on the type of diabetes, genetic predisposition, glycemic controls, and blood pressure. At the final stages of CKD, the kidneys fail completely and dialysis or a kidney transplant is needed.
We believe DM199 offers a potentially novel approach for the treatment CKD. CKD is a widespread health problem that generates significant economic burden throughout the world, including:
|
● |
30 million Americans and 120 million Chinese suffer from this debilitating and potentially life-threatening condition according to the National Kidney Foundation. |
|
● |
The primary causes of CKD are diabetes (Type 2 and Type 1) and hypertension. The Medical clinics of North America estimates that over 40% of those with Type 2 diabetes and 20% of those with Type 1 diabetes will eventually develop CKD, making it one of the more common risks for diabetics. |
|
● |
Patients with CKD are at greater risk for hypertension and heart disease. |
Currently, there is no cure for CKD and treatment involves management of the disease. Blood pressure medications, such as angiotensin converting enzyme inhibitors (“ACEi”) or angiotensin receptor blockers (“ARB”), are often prescribed to control hypertension, and hopefully, slow the progression of CKD. Nevertheless, according to the National Kidney Foundation, many patients continue to show declining kidney function, with the overall population having a lifetime risk of 3.6% of developing ESRD, where dialysis or a kidney transplant are needed.
Our Potential Treatments with DM199
Acute Ischemic Stroke
We believe that treatment of AIS with DM199 could have both immediate and long-term benefits for patients that could significantly improve outcomes following AIS. Immediate actions include activation of the KKS to release nitric oxide and improve microcirculation in ischemic tissue along with improvements in the balance between blood flow and brain activity (neurovascular coupling). Long-term (days following the stroke) actions include the restoration of the blood brain barrier through increases in T regulatory cells (“T-regs” – a subpopulation of T cells that modulate the immune system and prevent autoimmune disease) and inhibition of apoptotic cell death.
In China, a human urine-extracted KLKl protein (Kailikang®) is approved and marketed by Techpool Bio-Pharma Inc., a company controlled by Shanghai Pharmaceuticals Holding Co. Ltd. We believe Kailikang has been approved for the treatment of AIS in China with a treatment window of up to 48 hours post-stroke. More than 40 published clinical studies have demonstrated a beneficial effect of Kailikang treatment in AIS. According to a publication in the China Journal of Neurology, in a double-blinded, placebo-controlled trial of 446 patients treated with either KLK1 or a placebo administered up to 48 hours after a stroke showed significantly better scores on the European Stroke Scale and Activities of Daily Living at three weeks post-treatment and after three months using the Barthel Index. Furthermore, a comprehensive meta-analysis covering 24 clinical studies involving 2,433 patients published in the Journal of Evidenced Based Medicine concluded that human urinary KLK1 appears to ameliorate neurological deficits for patients with AIS and improves long-term outcomes, though a few treated patients suffered from transient hypotension.
As DM199 is a recombinant form of human KLK1, we believe it has the potential to preserve “at risk” brain tissue by increasing cerebral blood flow, establishing better collateral circulation, decreasing inflammation, reducing cell death, or apoptosis, and facilitating improved blood flow to at-risk ischemic penumbra brain tissue. We believe DM199 offers the potential for an improved recombinant product for worldwide use. We are developing DM199 to treat AIS patients with therapy beyond the current window of 3 to 4.5 hours for tissue plasminogen activator, or tPA, to up to 24 hours after the first sign of symptoms, thereby filling a large unmet need of patients who cannot receive tPA under the currently available treatment window of tPA. We believe this could potentially make therapy available to the millions of patients worldwide who currently have limited options regarding stroke therapy.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Chronic Kidney Disease
We believe DM199 has the potential to offer therapeutic benefits for CKD patients. The KLK1 protein also plays a vital role in normal kidney function, and BK and BK receptors are critical for kidney health and integrity. Patients with moderate to severe CKD often excrete abnormally low levels of KLK1 in their urine, leading to the hypothesis that this KLK1 deficit contributes to disease progression. We believe that DM199 may replenish endogenous KLK1 and activate the BK system that protects the kidney from damage. In fact, DM199 treatment in an animal model of Type 1 diabetes delayed the onset of the disease, attenuated the degree of insulitis (inflammation in the insulin producing islet cells of the pancreas) and improved pancreatic beta cell mass in a dose-dependent manner by increasing T-regs. By providing additional KLK1, DM199 has the following potentially beneficial actions:
|
● |
Improve blood flow to the kidney by restoring proper regulation of blood flow through veins arteries and especially capillaries (vasoregulation) |
|
● |
Support the structural integrity of the kidney by reducing scar tissue formation (fibrosis), oxidative stress, and inflammation. |
|
● |
Activate mechanisms that upregulate T-regs, improve insulin sensitization, glucose uptake and glycogen synthesis, and lower blood pressure. |
Further supporting the hypothesis that an intact KKS is critical for normal kidney function, a series of observational studies published in Immunopharmacology showed the amount of KLK1 released into the urine appears to be inversely correlated with the severity of disease in patients with CKD. Urinary KLK1 excretion was decreased in patients with both mild (not requiring dialysis) and severe (kidney failure/hemodialysis) renal disease compared to controls. The severity of the disease was negatively correlated with KLK1 excretion. Decreases in urinary KLK1 activity was seen especially when the reduction was associated with decreased glomerular filtration rate. We believe DM199 may potentially have advantages over ACEi because it restores already depleted KLK1 levels.
There is a significant need for alternative treatment strategies for CKD. We believe DM199 could potentially complement the use of ACEi or ARBs to improve kidney functions without increasing the risk for hyperkalemia, an abnormally high level of potassium in the blood. We believe DM199 may balance the renin-angiotensin system (“RAS”) that ACEi and ARBs work through by generating kinins to activate the BK receptors in the KKS. Activation of the BK receptors repair the renal system following CKD by improving vasodilation, anti-fibrosis, anti-inflammation, anti-oxidative stress, anti-thrombosis, and insulin sensitization. A significant differentiation between DM199 and ACEi and ARB treatment is that with DM199, hyperkalemia may be avoidable with a BK receptor-specific agonist.
Other Potential Programs
We are also currently developing a companion diagnostic, DMDx, to measure KLK1 levels. Several published studies indicate KLK1 insufficiency is associated with multiple disease states including hypertension, CKD and AIS. Measurements of endogenous KLK1 activity in both urine and plasma are inversely correlated with disease severity. Importantly, the decrease in urinary protein occurs in a disease state (e.g. CKD), where a primary hallmark is increased secretion of many other proteins. In this way, we believe KLK1 is a potentially unique diagnostic tool for such diseases.
We believe DM199 may also offer a potentially novel approach for the treatment of vascular dementia. Vascular dementia is caused by impaired blood supply to the brain, often caused by a stroke or a series of TIAs. KLK1 isolated from human urine has demonstrated the ability to improve cognitive function in vascular dementia patients and increase cerebral blood flow. According to the Alzheimer’s Society, one third of all stroke survivors could develop dementia within five years. We have drafted a protocol synopsis for a Phase 2 study in vascular dementia that we intend to initiate when we are sufficiently capitalized.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Our Competition and Current Treatments for Acute Ischemic Stroke and Chronic Kidney Disease
The biopharmaceutical industry is highly competitive and characterized by rapidly advancing technologies that focus on rapid development of proprietary drugs. We believe that our product candidates, development capabilities, experience and scientific knowledge provide us with competitive advantages. However, we face significant potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do, and experience in obtaining U.S. Food and Drug Administration (“FDA”) and other regulatory approvals of treatments and commercializing those treatments. Accordingly, our competitors may be more successful than us in obtaining approval for competitive products and achieving widespread market acceptance. Our competitors’ treatments may be more effectively marketed and sold than any products we may commercialize, thus limiting our market share and resulting in a longer period before we can recover the expenses of developing and commercializing our product candidates.
Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These activities may lead to consolidated efforts that allow for more rapid development of competitive product candidates.
We also compete for staff, development and clinical resources. These competitors may impair our ability to recruit or retain qualified scientific and management personnel, our ability to work with specific advisors, clinical contract organizations, due to conflicts of interest or capacity constraints, and may also delay recruitment of clinical study sites and study volunteers, impeding progress in our development programs.
We expect any products that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, price and the availability of reimbursement from government or other third-party payors. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are viewed as safer, more effective or less expensive than any products that we may develop.
Currently there is one approved pharmaceutical treatment for AIS. That treatment is tPA (Activase®), and its therapeutic window is limited to 3 to 4.5-hours after the AIS. There are, however, a number of companies that are actively pursuing a variety of approaches to develop pharmaceutical products for the treatment of acute ischemic stroke including, among others:
|
● |
Stem cells (Athersys, Inc.) |
|
● |
Cerebral edema (Biogen Inc.) |
|
● |
Anti-inflammatory and clot dissolving (Biogen Inc.) |
|
● |
Cell protection and anti-inflammation (ZZ Biotech LLC) |
|
● |
Inhibits platelet aggregation (Acticor Biotech SAS) |
In the United States, we are aware of one currently approved treatment for CKD. That treatment is an ACEi (Captopril®) which is approved for the treatment of patients with CKD caused by Type 1 diabetes. There are several pharmaceutical products for the treatment of CKD currently in clinical development, some of which include:
|
● |
Mineralcorticortisteroid receptor agonist (Bayer HealthCare Pharmaceuticals LLC) |
|
● |
CCR2 receptor antagonists (ChemoCentryx, Inc., Bristol-Myers Squibb Company) |
|
● |
Oxidative stress, cyclo-oxygenase 2 inhibitors (Reata Pharmaceuticals, Inc.) |
|
● |
Glycosylation inhibitors (Glycadia, Inc. aka Glycadia Pharmaceuticals) |
|
● |
Endothelin A receptor antagonists (AbbVie Inc.) |
|
● |
Cyclin nucleotide phosphodiesterase inhibitor (Pfizer Inc.) |
|
● |
Aldosterone receptor antagonists (Mitsubishi Tanabe Pharma Corporation) |
|
● |
Nitric oxide enzyme inhibitor (GenKyoTex SA) |
|
● |
Nitric oxide (Ironwood Pharmaceuticals, Inc.) |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Acute Ischemic Stroke
We believe that there is a large unmet therapeutic need for AIS treatments that can be administered beyond the 3 to 4.5-hour time window of tPA. With this large unmet therapeutic need, there is significant competition to develop new therapeutic options. New therapeutic options in development include tissue protection focused therapies (deliverable from hours to days after the stroke) that preserve and protect brain cells beyond the tPA therapeutic window. Currently, the most advanced treatments involve the mechanical removal of blood clots in brain arteries through sophisticated catheter-based approaches. According to published research, use of mechanical thrombectomy is growing and the window of time after a stroke where the procedure can be used is widening. These therapies are especially targeted toward preserving viable cells in the ischemic penumbra hours after a stroke. The goal is to provide treatment options for the vast majority of AIS patients who do not receive hospital care early enough to qualify for tPA therapy. We believe there is a very significant market opportunity for a drug that has a therapeutic window beyond that of tPA and is able to obtain regulatory approval.
Chronic Kidney Disease
Current treatment strategies for CKD include the strict control of high blood pressure and high blood sugar. The ACEi drug Captopril is approved for use in patients with CKD due to Type 1 diabetes and both ACEi and ARBs are widely prescribed to slow the progression of CKD. However, according to the National Kidney Foundation, 3.6% of the U.S. population over their lifetime will develop ESRD requiring dialysis or kidney transplantation. Furthermore, the treatment with ACEi and ARBs has been linked to hyperkalemia (elevated blood potassium levels), which increases the risk for abnormal heart rhythms and sudden death. In fact, two clinical trials investigating the use of ACEi and ARB combination therapy in kidney disease were stopped prematurely because participants developed hyperkalemia, or an abnormally high level of potassium in the blood. The added complication of hyperkalemia results in patients receiving suboptimal dosing or patients being untreated because they cannot tolerate the treatment. Additional side effects with ACEi treatment are angioedema (swelling of skin tissue) and persistent cough.
DM199 treatment is intended to directly replenish KLK1 levels, normalizing kidney function. Current treatment options, especially ACEi drugs, only partially restore kidney function and the association with high-risk side effects. While this increase benefits the kidney, ACEi drugs can generate excessive BK where is it not needed, potentially leading to related side effects such as cough and angioedema (swelling of skin and tissue). We believe DM199 treatment would potentially allow KLK1 to follow its normal physiological processes and release BK when and where it is needed, avoiding these side effects. Importantly, successful treatment with ACEi in kidney disease requires a fully functional KLK1 system, potentially making ACEi drugs less effective in patients with a pre-existing KLK1 deficit.
KLK1 derived from the pancreas of a pig, or porcine KLK1, is currently used to treat CKD in China and Japan. Porcine KLK1 is also used for hypertension and retinopathy in Japan, China and Korea. Over 20 clinical papers have been published in the Chinese literature supporting the therapeutic activity of porcine KLK1 given alone or combined with an ARB or an ACEi to treat CKD. These unblinded studies involve treating patients from a few weeks to six months in length and report improvement in kidney disease based on urinary albumin excretion rate (“UAER”) and other clinical endpoints of kidney disease. We believe that the combined results of these studies, which are consistent with our proposed mechanism of action for and preclinical studies of DM199, provide a good rationale for formal clinical development of DM199 for purposes of seeking approval for worldwide use as a potentially novel and ground-breaking drug treatment for CKD.
DM199 Clinical Studies
We have completed five clinical trials with DM199 in over 120 volunteers including multiple Phase 1 single dose ascending and multiple dose ascending studies in healthy volunteers and patients with Type 2 diabetes. Chronic dosing studies over 16 to 28 days were also conducted in healthy volunteers and patients with Type 2 diabetes. In all these studies, DM199 was well tolerated and demonstrated clear physiological activity. DM199 exhibited a favorable pharmacokinetic profile with extended half-life (i.e., the time required to reduce concentration of the drug in the body by one-half), supporting potential dosing intervals of up to one week. The dose limiting tolerability in healthy volunteers was orthostatic hypotension (a condition in which blood pressure falls significantly when a person stands) observed at dose levels much greater than those anticipated to be efficacious in patients. This is consistent with the DM199 mechanism of action and results observed in pre-clinical studies. Similarly, hypotension has been one prominent adverse event of high dose urinary KLK1 (Kailikang®). Two of our clinical studies have focused on patients with Type 2 diabetes. The first study enrolled ten (10) Type 2 diabetic patients. The patients were dosed with either DM199, at three single ascending dose levels, or placebo. DM199 was well-tolerated at all three dose levels by the diabetic patients with no dose limiting side effects. The second study in patients with type 2 diabetes enrolled 36 patients treated with two subcutaneous dose levels of DM199 over 28 days. This study achieved its primary endpoint and demonstrated that DM199 was well tolerated.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
In February 2018, we initiated treatment on the first patient in our Phase 2 REMEDY trial assessing the safety, tolerability and markers of therapeutic efficacy of DM199 in patients suffering from AIS. Our REMEDY trial is expected to enroll 60 patients to evaluate DM199 in patients with acute ischemic stroke (“AIS”). The study drug (DM199 or placebo) will be administered as an intravenous (“IV”) infusion within 24 hours of stroke symptom onset, followed by subcutaneous, or under the skin, injections once every 3 days for 21 days. The study is designed to measure safety and tolerability along with multiple tests designed to investigate DM199’s therapeutic potential including plasma-based biomarkers and standard functional stroke measures assessed at 90 days post-stroke. Standard functional stroke measurements include the Modified Rankin Scale (“MRS”), National Institutes of Health Stroke Scale (NIHSS), the Barthel Index (BI) and C-reactive protein (“CRP”), a measure of inflammation.
In March 2018, we had an in-person meeting with the Office of Drug Evaluation, Cardiovascular and Renal Division, of the FDA. The purpose of the meeting was to gain feedback and recommendations from the FDA on our planned clinical study of DM199 in patients with CKD. The study endpoints are expected to include:
|
● |
identifying dose(s) of DM199 that may normalize plasma concentrations of KLK1; |
|
● |
demonstrating safety and tolerability; and |
|
● |
evaluating standard measures of kidney function and treatment biomarkers. |
Based on the FDA’s guidance, we expect the study to include patients suffering from mild to moderate CKD (stage 3) due to Type 1 and Type 2 diabetes and will be designed to test multiple dosing strategies. Standard measures of safety, DM199 plasma levels and kidney function will be collected before, during and after DM199 treatment. We intend to file an Investigational New Drug (“IND”) application for this study in the second half of 2018. This study is intended to help identify the proper dosing strategy for future efficacy trials of DM199 for CKD.
In 2017, we completed and published in the International Journal of Clinical Trials the results from a Phase lb study with DM199 designed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy volunteers. Specifically, this study compared multiple doses levels of DM199, administered via intravenous (“IV”) and subcutaneous routes to identify a dose and delivery route that most closely compared to or improves upon the pharmacokinetic and pharmacodynamics profile of the approved urinary KLK1 in China. We found that a dose of DM199 administered via IV infusion mimicked the drug profile of IV-administered urinary derived KLK1 (Kailikang). We believe that this study also identified a dose of DM199, administered via subcutaneous injection, which had a superior pharmacokinetic profile and that maintained more normal KLK1 levels throughout day.
Potential DM199 Commercial Advantages
Several researchers have studied the structural and functional properties of KLK1. This deep body of knowledge has revealed the potential clinical benefits of KLK1 treatments. Today, forms of KLK1 derived from human urine and porcine pancreas are sold in Japan, China and Korea to treat acute ischemic stroke, chronic kidney disease, retinopathy, hypertension and related diseases. We are not aware of any synthetic version of KLK1 with regulatory approval for human use in any country, nor are we aware of any synthetic version in development besides our drug candidate DM199 (recombinant human KLK1).
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The growing understanding of KLK1’s role in human health and its use in Asia as an approved therapeutic highlight two important potential commercial advantages for DM199:
|
● |
KLK1 treatment is sold in Japan, China and Korea. Research has shown that patients with low levels of KLK1 are associated with a variety of diseases related to vascular dysfunction such as chronic kidney disease, acute ischemic strokes, retinopathy and hypertension. Clinical trial data with human urine and porcine KLK1 has demonstrated statistically significant clinical benefits of treating a variety of patients with KLK1 compared to placebo. These efficacy results are further substantiated by established markets in Japan, China and Korea for pharmaceutical sales of KLK1 derived from human urine and porcine pancreas. |
|
● |
KLK1 treatment has had limited side effects and has been well tolerated in studies to date. KLK1 is naturally produced by the human body; and therefore, the body’s own control mechanisms act to limit potential side effects. The only notable side effect observed in our clinical trials was orthostatic hypotension, or sudden drop in blood pressure, which was only seen at doses significantly higher than our anticipated therapeutic dose levels. Routine clinical use of KLK1 treatment in Asia has been well-tolerated by patients. In 2017, we completed a clinical trial comparing the pharmacokinetic profile of DM199 to Kailikang for acute ischemic stroke, which showed DM199, when administered in intravenous form, to have a profile similar to Kailikang. Further, when DM199 was administered subcutaneously, DM199 demonstrated a superior, longer acting, pharmacokinetic profile to Kailikang. |
We have conducted numerous internal and third-party analyses to demonstrate that DM199 is structurally and functionally equivalent to KLK1 derived from human urine. The amino acid structure of DM199 is identical to the human urine form, and the enzymatic and pharmacokinetic profiles are substantially similar to both human urinary and porcine derived KLK1. The physiological effects of DM199 on blood pressure, from our completed studies, mirror that of human urinary and porcine-derived forms of KLK1. We believe that the results of this work suggest that the therapeutic action of DM199 will be the same or better than that of the forms marketed in Asia. In addition, there are also significant formulation, manufacturing and other advantages for our synthetic human KLK1 drug candidate DM199:
|
● |
Potency and Impurity Considerations. KLK1 derived from human urine or porcine pancreas may contain impurities, endotoxins, and chemical byproducts due to the inherent variability of the isolation and purification process. We believe that this creates the risk of inconsistencies in potency and impurities from one production run to the next. However, we expect to produce a consistent formulation of KLK1 that is free of endotoxins and other impurities, which we believe will provide therapeutic benefits. |
|
● |
Cost and Scalability. Large quantities of human urine and porcine pancreas must be obtained to derive a small amount of KLK1. This creates potential procurement, cost and logistical challenges to source the necessary raw organic material, particularly for human urine sourced KLK1. Once sourced, the raw organic material is processed using chemicals and costly capital equipment and produces a significant amount of byproduct waste. Our novel recombinant manufacturing process utilizes widely available raw materials and can be readily scaled for commercial production. Accordingly, we believe our manufacturing process has significant cost and scalability advantages. |
|
● |
Regulatory. We are not aware of any attempts by manufacturers of the urine or porcine based KLK1 products to pursue regulatory approvals in the United States. We believe that this is related to challenges presented by using inconsistent and potentially hazardous biomaterials, such as human urine and porcine pancreas, and their resulting ability to produce consistent drug product. Our novel recombinant manufacturing process utilizes widely available raw materials which we believe provides a significant regulatory advantage, particularly in regions such as the United States, Europe and Canada, where safety standards are high. |
Regulatory Approval
Securing regulatory approval for the manufacture and sale of human therapeutic products in the United States, Europe, Canada and other commercial territories is a long and costly process that is controlled by that particular territory’s national regulatory agency. The national regulatory agency in the United States is the FDA, in Europe it is the European Medicines Agency (“EMA”), and in Canada it is Health Canada. Other national regulatory agencies have similar regulatory approval processes, but each national regulatory agency has its own approval processes. Approval in the United States, Europe or Canada does not assure approval by other national regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Prior to obtaining regulatory approval to market a drug product, every national regulatory agency has a variety of statutes and regulations which govern the principal development activities. These laws require controlled research and testing of products, governmental review, and approval of a submission containing preclinical and clinical data establishing the safety and efficacy of the product for each use sought, approval of manufacturing facilities including adherence to good manufacturing practices (“GMP”) during production and storage, and control of marketing activities, including advertising, labeling and pricing approval.
None of our product candidates have been completely developed or tested; and, therefore, we are not yet in a position to seek regulatory approval in any territory to market any of our product candidates.
The clinical testing, manufacturing, labeling, storage, distribution, record keeping, advertising, promotion, import, export, and marketing, among other things, of our product candidates are subject to extensive regulation by governmental authorities in the United States and other countries. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable requirements at any time during the product development process, approval process, or after approval may subject us to a variety of administrative or judicial sanctions, including refusal by the applicable regulatory authority to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by the FDA and the Department of Justice or other governmental entities.
U.S. Approval Process
In the United States, the FDA, a federal government agency, is responsible for the drug approval process. The FDA’s mission is to ensure that all medications on the market are safe and effective. The FDA’s approval process examines potential drugs; and only those that meet strict requirements are approved.
The U.S. food and drug regulations require licensing of manufacturing facilities, carefully controlled research and testing of products, governmental review and approval of test results prior to marketing of therapeutic products, and adherence to GMP, as defined by each licensing jurisdiction, during production.
The drug approval process begins with the discovery of a potential drug. Pharmaceutical companies then test the drug extensively. A description of the different stages in the drug approval process in the United States follows.
Stage 1: Preclinical Research. After an experimental drug is discovered, research is conducted to help determine its potential for treating or curing an illness. This is called preclinical research. Animal and/or bench studies are conducted to determine if there are any harmful effects of the drug and to help understand how the drug works. Information from these experiments is submitted to the FDA in an IND. The FDA reviews the information in the IND and decides if the drug is safe to study in humans.
Stage 2: Clinical Research. In Stage 2, the experimental drug is studied in humans. The studies are known as clinical trials. Clinical trials are carefully designed and controlled experiments in which the experimental drug is administered to patients to test its safety and to determine the effectiveness of an experimental drug. The four general phases of clinical research are described below.
Phase I Clinical Studies. Phase I clinical studies are generally conducted with healthy volunteers who are not taking other medicines; patients with the illness that the drug is intended to treat are not tested at this stage. Ultimately, Phase I studies demonstrate how an experimental drug affects the body of a healthy individual. Phase I consists of a series of small studies consisting of “tens” of volunteers. Tests are done on each volunteer throughout the study to see how the person’s body processes, responds to, and is affected by the drug. Low doses and high doses of the drug are usually studied, resulting in the determination of the safe dosage range in volunteers by the end of Phase I. This information will determine whether the drug proceeds to Phase II.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Phase II Clinical Studies. Phase II clinical studies are conducted in order to determine how an experimental drug affects people who have the disease to be treated. Phase II usually consists of a limited number of studies that help determine the drug’s short-term safety, side effects, and general effectiveness. The studies in Phase II often are controlled investigations involving comparison between the experimental drug and a placebo, or between the experimental drug and an existing drug. Information gathered in Phase II studies will determine whether the drug proceeds to Phase III.
Phase III Clinical Studies. Phase III clinical studies are expanded controlled and uncontrolled trials that are used to more fully investigate the safety and effectiveness of the drug. These trials differ from Phase II trials because a larger number of patients are studied (sometimes in the thousands) and because the studies are usually of longer duration. As well, Phase Ill studies can include patients who have more than one illness and are taking medications in addition to the experimental drug used in the study. Therefore, the patients in Phase III studies more closely reflect the general population. The information from Phase III forms the basis for most of the drug’s initial labeling, which will guide physicians on how to use the drug.
Phase IV Clinical Studies. Phase IV clinical studies are conducted after a drug is approved. Companies often conduct Phase IV studies to more fully understand how their drug compares to other drugs. Also, the FDA may require additional studies after the drug is approved. FDA-required Phase IV studies often investigate the drug in specific types of patients that may not have been included in the Phase III studies and can involve very large numbers of patients to further assess the drug’s safety.
Stage 3: FDA Review for Approval. Following Phase III, the pharmaceutical company prepares reports of all studies conducted on the drug and a complete dossier on the manufacturing of the product and submits the reports to the FDA in a New Drug Application (“NDA”). The FDA reviews the information in the NDA to determine if the drug is safe and effective for its intended use. Occasionally, the FDA will ask experts for their opinion of the drug. If the FDA determines that the drug is safe and effective, the drug will be approved.
Stage 4: Marketing. After the FDA has approved the experimental drug, the pharmaceutical company can make it available to physicians and their patients. A company also may continue to conduct research to discover new uses for the drug. Each time a new use for a drug is discovered, the drug once again is subject to the entire FDA approval process before it can be marketed for that purpose.
Any pharmaceutical products for which FDA approvals are obtained are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, promoting pharmaceutical products for uses or in patient populations that are not described in the pharmaceutical product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties.
The FDA also may require post-marketing testing, known as Phase IV testing, risk evaluation and mitigation strategies and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
European Approval Process
The EMA is roughly parallel to the U.S. FDA in terms of the drug approval process and the strict requirements for approval. The EMA was set up in 1995 in an attempt to harmonize, but not replace, the work of existing national medicine regulatory bodies in individual European countries. As with the FDA, the EMA drug review and approval process follows different stages from preclinical testing through clinical testing in Phase I, II, and III. There are some differences between the FDA and EMA review process, specifically the review process in individual European countries. Such differences may allow certain drug products to be tested in patients at an earlier stage of development.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services and other divisions of the U.S. government, including, the Department of Health and Human Services, the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, if a drug product is reimbursed by Medicare, Medicaid, or other federal or state healthcare programs, our company, including our sales, marketing and scientific/educational grant programs, must comply with the federal False Claims Act, as amended, the federal Anti-Kickback Statute, as amended, and similar state laws. If a drug product is reimbursed by Medicare or Medicaid, pricing and rebate programs must comply with, as applicable, the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 (“OBRA”), and the Medicare Prescription Drug Improvement and Modernization Act of 2003. Among other things, OBRA requires drug manufacturers to pay rebates on prescription drugs to state Medicaid programs and empowers states to negotiate rebates on pharmaceutical prices, which may result in prices for our future products that will likely be lower than the prices we might otherwise obtain. Additionally, the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, “PPACA”), substantially changes the way healthcare is financed by both governmental and private insurers. There may continue to be additional proposals relating to the reform of the U.S. healthcare system, in the future, some of which could further limit coverage and reimbursement of drug products. If drug products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements may apply.
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of coverage and adequate reimbursement from third-party payers, including government health administrative authorities, managed care providers, private health insurers and other organizations. In the United States, private health insurers and other third-party payers often provide reimbursement for products and services based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such treatments. Third-party payers are increasingly examining the medical necessity and cost-effectiveness of medical products and services in addition to their safety and efficacy; and, accordingly, significant uncertainty exists as to the coverage and reimbursement status of newly approved therapeutics. In particular, in the United States, the European Union and other potentially significant markets for our product candidates, government authorities and third-party payers are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which has resulted in lower average selling prices. Further, the increased emphasis on managed healthcare in the United States and on country and regional pricing and reimbursement controls in the European Union will put additional pressure on product pricing, reimbursement and usage, which may adversely affect our future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general. As a result, coverage and adequate third party reimbursement may not be available for our products to enable us to realize an appropriate return on our investment in research and product development.
The market for our product candidates for which we may receive regulatory approval will depend significantly on access to third-party payers’ drug formularies or lists of medications for which third-party payers provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical companies. Also, third-party payers may refuse to include a particular branded drug in their formularies or may otherwise restrict patient access to a branded drug when a less costly generic equivalent or another alternative is available. In addition, because each third-party payer individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time-consuming and costly process. We would be required to provide scientific and clinical support for the use of any product candidate to each third-party payer separately with no assurance that approval would be obtained, and we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our product candidates. This process could delay the market acceptance of any of our product candidates for which we may receive approval and could have a negative effect on our future revenues and operating results. We cannot be certain that our product candidates will be considered cost-effective. If we are unable to obtain coverage and adequate payment levels for our product candidates from third-party payers, physicians may limit how much or under what circumstances they will prescribe or administer them and patients may decline to purchase them. This in turn could affect our ability to successfully commercialize our products and impact our profitability, results of operations, financial condition, and future success.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Research and Development
We have devoted substantially all of our efforts to research and development which therefore comprises the largest component of our operating costs. Our primary focus over the past approximately eight years has been our lead product candidate, DM199, which is currently in clinical development for AIS and is expected to commence clinical development for CKD in the second half of 2018 or first half of 2019.
We expect our research and development expenses will continue to increase in the future as we advance our initial product candidate through clinical trials in AIS and CKD and seek to expand our product candidate portfolio. The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming and we consider the active management and development of our clinical pipeline to be integral to our long-term success. The actual probability of success for each product candidate, clinical indication and preclinical program may be affected by a variety of factors including, among other things, the safety and efficacy data for product candidates, amounts invested in the program, competition and competitive developments, manufacturing capability and commercial viability.
Research and development expenses include:
|
● |
expenses incurred under contract research agreements and other agreements with third parties; |
|
● |
expenses incurred under agreements with clinical trial sites that conduct research and development activities on our behalf; |
|
● |
employee and consultant-related expenses, which include salaries, benefits, travel and share-based compensation; |
|
● |
laboratory and vendor expenses related to the execution of clinical trials and non-clinical studies; |
|
● |
the cost of acquiring, developing, manufacturing, and distributing clinical trial materials; and |
|
● |
facilities and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance, and other supply costs. |
Research and development costs are expensed as incurred. Costs for certain development activities such as clinical trials are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites.
We expect that it will be several years, if ever, before we have any product candidates ready for commercialization. Our research and development expenses totaled $3.2 million and $1.7 million in 2017 and 2016, respectively.
Manufacturing
We do not own or operate manufacturing facilities for the production of our product candidates nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, active pharmaceutical ingredients and finished drug product for our clinical trials. We do not have any current contractual arrangements for the manufacture of commercial supplies of any product candidates. We currently employ internal resources and third-party consultants to manage our manufacturing contractors.
Sales and Marketing
We have not yet defined our sales, marketing or product distribution strategy for our initial product candidate, or any future product candidates, because it is still early in the clinical development stage. We currently expect to partner with a large pharmaceutical company for sales execution. However, our future commercial strategy may include the use of distributors, a contract sales force or the establishment of our own commercial and specialty sales force, as well as similar strategies for regions and territories outside the United States.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Intellectual Property
We view patents and other means of intellectual property protection including trade secrets as an important component of our core business. We focus on translating our innovations into intangible property protecting our proprietary technology from infringement by competitors. To that end, patents are reviewed frequently and continue to be sought in relation to those components or concepts of our preclinical and clinical products to provide protection. Our strategy, where possible, is to file patent applications to protect our product candidates, as well as methods of manufacturing, administering and using a product candidate. Prior art searches of both patent and scientific databases are performed to evaluate novelty, inventiveness and freedom-to-operate. We require all employees, consultants, and parties to sign a collaborative research agreement and to execute confidentiality agreements upon the commencement of employment, consulting relationships, or a collaboration with us. These agreements require that all confidential information developed or made known during the course of the engagement with us is to be kept confidential. We also maintain agreements with our scientific staff and all parties contracted in a scientific capacity affirming that all inventions resulting from work performed for us, using our property, or relating to our business and conceived or completed during the period covered by the agreement are the exclusive property of our company.
Our patent portfolio includes patents and pending applications that are owned by us, which include claims for composition of matter and methods of use. For our DM199 program, this includes two patent families that are directed to composition of matter, and methods of use.
The DM199 patents protect composition of matter including glycoforms, formulations, methods of administration and a variety of therapeutic approaches pertaining to current and future indications. All intellectual property associated with development, manufacturing and testing of DM199 in disease models is owned by us. We currently have additional patent applications for DM199. Additionally, for the manufacture of DM199, we have licensed an expression system and cell line with proven GMP and regulatory support and are contracting with a contract manufacturing organization (“CMO”) with proven GMP experience in manufacturing of recombinant proteins for clinical trials.
Our DM199 patent portfolio includes granted U.S. and worldwide patents as well as one U.S. and worldwide pending application. Granted and pending claims offer various forms of protection for DM199 including claims to compositions of matter, pharmaceutical compositions, specific formulations and dosing levels, and methods for treating a variety of diseases, including stroke, chronic kidney disease, and related disorders. These U.S. patents and applications, and their foreign equivalents, are described in more detail below.
Issued patents held by us cover the DM199 composition of matter based on an optimized combination of closely-related isoforms that differ in the extent of glycosylation (process by which sugars are chemically attached to proteins). This patent family covers the most pharmacologically active variants of DM199 as wells as the vectors and reagents used to manufacture DM199 at scale and is due to expire in 2033. A second issued U.S. patent covers all standard formulations and delivery methods including injectable, oral and other novel technologies. A pending U.S. and worldwide patent covers a range of dose levels and dosing regimens of DM199 useful for treating a wide range of diseases associated with microvascular dysfunction (e.g. pulmonary hypertension, endothelial dysfunction, cardiovascular disease, chronic kidney disease, metabolic disorder including diabetes, obesity, stroke, and vascular dementia).
Methods and reagents required for commercial scale manufacture of DM199 are subject to a series of patents issued to our manufacturing partner. We exclusively license these patents from our manufacturing partner for the production of DM199 or any human KLK1 protein. The large-scale manufacturing of KLK1 has been attempted by multiple pharmaceutical and commercial entities without success and we believe that our proprietary technology along with trade secrets will provide substantial protection from third-party competitors.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We believe that the most relevant granted U.S. patents with composition of matter or method of use claims covering DM199 are listed below, along with their projected expiration dates exclusive of any patent term extension.
|
Patent Number |
Title |
Geography |
Type |
Expiration |
||||
|
Issued patents |
||||||||
|
US 9,364,521 |
Human Tissue Kallikrein 1 Glycosylation Isoforms |
US/Europe |
Composition of matter |
2033 |
||||
|
US 9,616,015 |
Formulations for Human Tissue Kallikrein-1 for Parenteral Delivery and Related Methods |
US |
Method of use |
2033 |
||||
|
Pending applications |
||||||||
|
PCT/US2018/021749 |
Dosage Forms of Tissue Kallikrein 1 |
US/Worldwide |
Method of use |
2037 |
||||
Employees
As of August 15, 2018, we had 12 full-time employees. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements. We believe our employee relations are good.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and, for so long as we are an emerging growth company, are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. These include, but are not limited to:
|
● |
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; |
|
● |
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
|
● |
reduced disclosure obligations regarding executive compensation; and |
|
● |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We have determined to opt out of the exemption from compliance with new or revised financial accounting standards. Our decision to opt out of this exemption is irrevocable. We have elected to adopt the reduced disclosure requirements available to emerging growth companies. As a result of these elections, the information that we provide in this registration statement may be different than the information you may receive from other public companies in which you hold equity interests.
We may remain an emerging growth company until the last day of the fiscal year following the fifth anniversary of our first sale of common shares pursuant to a registration statement under the Securities Act or until such earlier time as we have more than $1.07 billion in annual revenue, the market value of our common shares held by non-affiliates is more than $700 million or we issue more than $1 billion of non-convertible debt over a three-year period.
Enforceability of Civil Liabilities Against Foreign Persons
We are organized under and governed by the federal laws of Canada, and, accordingly, are governed by the applicable laws of Canada. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon the U.S. federal securities laws or the securities laws or “blue sky” laws of any state within the United States and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws or any such state securities laws or blue sky laws. Accordingly, it may not be possible to enforce judgments obtained in the United States against us.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Corporate Information
We were incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of continuance dated January 21, 2000. We changed our name to DiaMedica Inc. on February 26, 2001. On April 11, 2016, our articles of continuance were amended to continue our company from The Corporations Act (Manitoba) to the Canada Business Corporations Act. On December 8, 2016, we changed our name to DiaMedica Therapeutics Inc.
Our registered office is located at 301-1665 Ellis Street, Kelowna, British Columbia, V1Y 2B3 and our principal executive office is located at our wholly owned subsidiary, DiaMedica USA Inc., located at 2 Carlson Parkway, Suite 260, Minneapolis, Minnesota, USA 55447. Our telephone number is 763-312-6755. Our internet website address is http://www.diamedica.com. Information contained on our website does not constitute part of this registration statement.
ITEM 1.A. RISK FACTORS
An investment in our common shares involves a high degree of risk and should be considered speculative. An investment in our common shares should only be undertaken by those persons who can afford the total loss of their investment. You should consider carefully the risks and uncertainties described below, as well as other information contained in this registration statement. The risks and uncertainties below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we believe to be immaterial may also adversely affect our business. If any of the following risks occur, our business, financial condition, and results of operations could be seriously harmed and you could lose all or part of your investment. Further, if we fail to meet the expectations of the public market in any given period, the market price of our common shares could decline.
We operate in a highly competitive environment that involves significant risks and uncertainties, some of which are outside of our control and some of which are inherent in the biotechnology industry.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred substantial losses since our inception and expect to incur future losses and may never become profitable.
We are a clinical stage biopharmaceutical company focused on the development of novel recombinant proteins. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate will fail to prove effective, gain regulatory approval or become commercially viable. We do not have any products approved by regulatory authorities and have not generated any revenues from collaboration and licensing agreements or product sales to date, and have incurred significant research, development and other expenses related to our ongoing operations and expect to continue to incur such expenses. As a result, we have not been profitable and have incurred significant operating losses in every reporting period since our inception. For the three months ended March 31, 2018, we incurred a net loss of $650,000 and for the years ended December 31, 2017 and 2016, we incurred a net loss of $4.3 million and $2.2 million, respectively. As of March 31, 2018, we had an accumulated deficit of $40.9 million. Our operating losses are expected to increase in the near term as we continue our product development efforts and are expected to continue until such time as any future product sales, royalty payments, licensing fees, and/or milestone payments are sufficient to generate revenues to fund our continuing operations. In addition, we expect to our operating expenses to increase compared to last year as a result of our anticipated U.S. public reporting company status. We are unable to predict the extent of any future losses or when we will become profitable, if ever. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
There is substantial doubt about our ability to continue as a going concern.
The report of our independent registered public accounting firm on our December 31, 2017 audited consolidated financial statements includes an explanatory paragraph referring to our ability to continue as a going concern. As of December 31, 2017 and March 31, 2018, we had cash balances of approximately $1.4 million and $6.7 million, respectively. In addition, we had outstanding accounts payable and accrued liabilities of $919,000 and $1.1 million as of December 31, 2017 and March 31, 2018, respectively. On March 29, 2018, we completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to March 19, 2020 and March 29, 2020 for the first and second tranches, respectively, subject to earlier expiration under certain conditions. Additional funding will be required to continue our research and development and other operating activities as we have not reached successful commercialization of our product candidates. These circumstances cast significant doubt as to our ability to continue as a going concern.
We will require additional funds to finance our operations, which may not be available to us on acceptable terms, or at all. As a result, we may not complete the development and commercialization of our product candidates or develop new product candidates.
We require significant additional funds for further research and development, planned clinical trials, and the regulatory approval process. We may raise additional funds for these purposes through public or private equity or debt financing, or through collaborations with other biotechnology companies, or financing from other sources may be undertaken. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. Debt financing, if available, may involve agreements that include conversion discounts or covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, or strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. It is possible that financing will not be available or, if available, may not be on favorable terms. The availability of financing will be affected by the results of scientific and clinical research; the ability to attain regulatory approvals; market acceptance of our product candidates; the state of the capital markets generally with particular reference to pharmaceutical, biotechnology, and medical companies; the status of strategic alliance agreements; and other relevant commercial considerations. If adequate funding is not available, we may be required to implement cost reduction strategies; delay, reduce, or eliminate one or more of our product development programs; relinquish significant rights to product candidates or obtain funds on less favorable terms than we would otherwise accept; and/or divest assets through a merger, sale, or liquidation of our company.
We currently have no product revenue and will not be able to maintain our operations and research and development without additional funding.
To date, we have primarily relied on equity financing to fund our working capital requirements and drug development activities. A substantial amount of additional capital is needed to develop our product candidates to a point where they may be commercially sold. Our future operations are dependent upon our ability to generate product sales, negotiate collaboration or license agreements, obtain research grant funding, defer expenditures or other strategic alternatives, and/or secure additional funds. While we are striving to achieve these plans, there is no assurance these and other strategies will be achieved or that such sources of funds will be available or obtained on favorable terms or obtained at all. Our ability to continue as a going concern is dependent on our ability to continue obtaining sufficient funds to conduct our research and development, and to successfully commercialize our product candidates.
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates.
We may be adversely affected by foreign currency fluctuations. To date, we have been primarily funded through issuances of equity and proceeds from the exercise of warrants and stock options, which are denominated both in Canadian and U.S. dollars. Also, a portion of our expenditures are in U.S. dollars, Canadian dollars, British pounds, and Australian dollars, and, therefore, we are subject to foreign currency fluctuations which may, from time to time, impact our financial position and results of operations.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Risks Related to our Business and our Industry
Our prospects depend on the success of our product candidates which are at early stages of development, and we may not generate revenue for several years, if at all, from those products.
We have compounds in various stages of development, but we currently have no product candidates beyond Phase II clinical trials. Prior to commercialization of any potential product, significant additional investments will be necessary to complete the development of any of our product candidates. Preclinical and clinical trial work must be completed before some of our product candidates could be ready for use within the markets that we have identified. We may fail to develop any products, to obtain regulatory approvals, to enter clinical trials, or to commercialize any products. Competitors may develop alternative products and methodologies to treat and diagnose the disease indications we are pursuing, thus reducing our competitive advantages. We do not know whether any of our product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost, or successfully marketed. The product candidates we are currently developing are not expected to be commercially viable for several years and we may encounter unforeseen difficulties or delays in commercializing our product candidates. In addition, our product candidates may cause undesirable side effects. Results of early preclinical research may not be indicative of the results that will be obtained in later stages of preclinical or clinical research. If regulatory authorities do not approve our product candidates or if we fail to maintain regulatory compliance, we would have limited ability to commercialize our product candidates, and our business and results of operations would be harmed. If we do succeed in developing products, we will face many potential obstacles such as the need to develop or obtain manufacturing, marketing, and distribution capabilities.
We rely and will continue to rely on third parties to plan, conduct, and monitor our preclinical and clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring, and project management. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs may face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, cancelled, or rendered ineffective.
We are in litigation with a contract research organization seeking to compel them to comply with the terms of a clinical trial research agreement and their failure to perform as required could adversely affect our ability to obtain regulatory approval for a new drug.
In March 2013, we entered into a clinical research agreement with a contract research organization to perform a double-blinded, placebo-controlled, single-dose and multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and proof of concept of DM199 in healthy subjects and patients with type 2 diabetes mellitus. In one arm of this study, we enrolled 36 patients with type 2 diabetes who were treated with two subcutaneous dose levels of DM199 over a 28-day period. This study achieved its primary endpoint and demonstrated that DM199 was well tolerated. The secondary efficacy endpoints were confounded due to what we believe were serious execution errors by the contract research organization that conducted the study. To date, we have been unable to obtain the complete study records from the contract research organization and generate a final study report. The lack of a final study report may delay our ability to obtain the acceptance of an investigational new drug application in the United States, which would delay or prevent us from conducting clinical development or obtaining approval in the United States. We have initiated litigation with the contract research organization to compel them to comply with the term of the clinical research agreement, including providing full study records, and to recover damages. Litigation distracts the attention of our management from our business, is expensive and the outcome is uncertain.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We rely on contract manufacturers over whom we have limited control. If we are subject to quality, cost, or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm.
We rely on CMOs to manufacture our product candidates for our preclinical studies and clinical trials. We rely on CMOs for manufacturing, filling, packaging, storing, and shipping of drug product in compliance with current GMP regulations applicable to our product candidates. The FDA ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with GMP regulations. The GMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packing of a drug product.
There can be no assurances that CMOs will be able to meet our timetable and requirements. If we are unable to arrange for alternative third-party manufacturing sources on commercially reasonable terms or in a timely manner, we may be delayed in the development of our product candidates. Further, contract manufacturers must operate in compliance with GMP and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon third parties for the manufacture of our products may adversely affect our ability to develop products on a timely and competitive basis and, if we are able to commercialize our product candidates, may adversely affect our profit margins.
If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we would incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct preclinical studies in animals and extensive clinical trials in humans to demonstrate the safety and efficacy of our product candidates. Clinical testing is expensive and difficult to design and implement, can take many years to complete, and has uncertain outcomes. The outcome of preclinical studies and early clinical trials may not predict the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety profiles, notwithstanding promising results in earlier trials. We do not know whether the clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market any of our product candidates in any jurisdiction. A product candidate may fail for safety or efficacy reasons at any stage of the testing process. A major risk we face is the possibility that none of our product candidates under development will successfully gain market approval from the FDA or other regulatory authorities, resulting in us being unable to derive any commercial revenue from them after investing significant amounts of capital in multiple stages of preclinical and clinical testing.
If we experience delays in clinical testing, we will be delayed in commercializing our product candidates, and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, or at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The commencement and completion of clinical trials for our products may be delayed for a number of reasons, including delays related, but not limited, to:
|
● |
failure by regulatory authorities to grant permission to proceed or placing the clinical trial on hold; |
|
● |
patients failing to enroll or remain in our trials at the rate we expect; |
|
● |
suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with GMP requirements; |
|
● |
any changes to our manufacturing process that may be necessary or desired; |
|
● |
delays or failure to obtain clinical supply from contract manufacturers of our products necessary to conduct clinical trials; |
|
● |
product candidates demonstrating a lack of safety or efficacy during clinical trials; |
|
● |
patients choosing an alternative treatment for the indications for which we are developing any of our product candidates or participating in competing clinical trials; |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
● |
patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects, or other reasons; |
|
● |
reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
|
● |
competing clinical trials and scheduling conflicts with participating clinicians; |
|
● |
clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
|
● |
failure of our contract research organizations (“CROs”) to satisfy their contractual duties or meet expected deadlines; |
|
● |
inspections of clinical trial sites by regulatory authorities, Institutional Review Boards (“IRBs”) or ethics committees finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
|
● |
one or more IRBs or ethics committees rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
|
● |
failure to reach agreement on acceptable terms with prospective clinical trial sites. |
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees for re-examination, which may impact the cost, timing or successful completion of that trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition, and prospects.
We may not be able to file INDs to commence additional clinical trials on the timelines we expect, and even if we are able to, the FDA may not permit us to proceed in a timely manner, or at all.
Prior to commencing clinical trials in the United States for any of our product candidates, we may be required to have an allowed IND for each product candidate and to file additional INDs prior to initiating any additional clinical trials for DM199. We believe that the data from previous preclinical studies will support the filing of additional INDs to enable us to undertake additional clinical studies as we have planned. However, submission of an IND may not result in the FDA allowing further clinical trials to begin and, once begun, issues may arise that will require us to suspend or terminate such clinical trials. Additionally, even if relevant regulatory authorities agree with the design and implementation of the clinical trials set forth in an IND, these regulatory authorities may change their requirements in the future. Failure to submit or have effective INDs and commence clinical programs will significantly limit our opportunity to generate revenue.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting stroke patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials on a timely basis or at all. The factors that affect our ability to enroll patients are largely uncontrollable and include, but are not limited to, the following:
|
● |
size and nature of the patient population; |
|
● |
eligibility and exclusion criteria for the trial; |
|
● |
design of the study protocol; |
|
● |
competition with other companies for clinical sites or patients; |
|
● |
the perceived risks and benefits of the product candidate under study; |
|
● |
the patient referral practices of physicians; and |
|
● |
the number, availability, location, and accessibility of clinical trial sites. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Regulatory approval processes are lengthy, expensive, and inherently unpredictable. Our inability to obtain regulatory approval for our product candidates would substantially harm our business.
Our shareholders and other investors should be aware of the risks, problems, delays, expenses, and difficulties which we may encounter in light of the extensive regulatory environment within which our business is carried out. Numerous statutes and regulations govern the manufacture and sale of non-therapeutic and human therapeutic products in the United States, European Union, Canada and other countries that are the intended markets for our products and product candidates. Such legislation and regulation governs the approval of manufacturing facilities, the testing procedures, and controlled research that must be carried out, and the preclinical and clinical data that must be collected prior to marketing approval. Our research and development efforts, as well as any future clinical trials, and the manufacturing and marketing of any products we may develop, will be subject to and restricted by such extensive regulation.
The process of obtaining necessary regulatory approvals is lengthy, expensive, and uncertain. We or our future collaborators may fail to obtain the necessary approvals to commence or continue clinical testing or to manufacture or market our potential products in reasonable time frames, if at all. In addition, governmental authorities in the United States or other countries may enact regulatory reforms or restrictions on the development of new therapies that could adversely affect the regulatory environment in which we operate or the development of any products we may develop.
Completing clinical testing and obtaining required approvals is expected to take several years and to require the expenditure of substantial resources. There can be no assurance that clinical trials will be completed successfully within any specified period of time, if at all. Furthermore, clinical trials may be delayed or suspended at any time by us or by the various regulatory authorities if it is determined at any time that the subjects or patients are being exposed to unacceptable risks.
Any failure or delay in obtaining regulatory approvals would adversely affect our ability to utilize our technology and would therefore adversely affect our operations. Furthermore, no assurance can be given that our product candidates will prove to be safe and effective in clinical trials or that they will receive the requisite regulatory approval. Moreover, any regulatory approval of a drug which is eventually obtained may be granted with specific limitations on the indicated uses for which that drug may be marketed. Furthermore, product approvals may be withdrawn if problems occur following initial marketing or if compliance with regulatory standards is not maintained.
In addition, we rely to some extent on the availability of certain agents that are currently marketed by other firms. Such agents may become unavailable as a result of failing to meet regulatory requirements.
We may not achieve our publicly announced milestones according to schedule, or at all.
From time to time, we may announce the timing of certain events we expect to occur, such as the anticipated timing of results from our clinical trials. These statements are forward-looking and are based on the best estimates of management at the time relating to the occurrence of such events. However, the actual timing of such events may differ from what has been publicly disclosed. The timing of events such as the initiation or completion of a clinical trial, filing of an application to obtain regulatory approval, or an announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a CMO or a CRO or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business plan, financial condition or operating results, and the trading price of our common shares.
We face competition from other biotechnology and pharmaceutical companies and our financial condition and operations will suffer if we fail to effectively compete.
Technological competition is intense in the industry in which we operate. Competition comes from pharmaceutical companies, biotechnology companies, and universities, as well as companies that offer non-pharmaceutical solutions in the markets we may attempt to address with our products. Many of our competitors have substantially greater financial and technical resources; more extensive research and development capabilities; and greater marketing, distribution, production, and human resources than we do. Moreover, competitors may develop products more quickly than us and may obtain regulatory approval for such products more rapidly than we do. Products and processes which are more effective than those that we intend to develop may be developed by our competitors. Research and development by others may render our product candidates non-competitive or obsolete.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We heavily rely on the capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
We depend on our management personnel. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical, and regulatory personnel, particularly as we expand our activities and seek regulatory approvals for clinical trials. We enter into agreements with our scientific and clinical collaborators and advisors, key opinion leaders, and academic partners in the ordinary course of our business. We also enter into agreements with physicians and institutions who will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officers or other key personnel could potentially harm our business, operating results, or financial condition.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we have established, comply with federal and state health-care fraud and abuse laws and regulations, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions.
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We have in the past and may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations, or in-licensing one or more product candidates. Acquisitions, collaborations and in-licenses involve numerous risks, including, but not limited to:
|
● |
substantial cash expenditures; |
|
● |
technology development risks; |
|
● |
potentially dilutive issuances of equity securities; |
|
● |
incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
|
● |
difficulties in assimilating the operations of the acquired companies; |
|
● |
potential disputes regarding contingent consideration; |
|
● |
diverting our management’s attention away from other business concerns; |
|
● |
entering markets in which we have limited or no direct experience; and |
|
● |
potential loss of our key employees or key employees of the acquired companies or businesses. |
We cannot provide assurance that any acquisition, collaboration, or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the growth associated with some of these acquisitions, collaborations and in-licenses. We cannot provide assurance that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our product candidates may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors, or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect our share price and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
We may not be able to reproduce the results of previously conducted clinical studies and/or comparisons to other forms of KLK1, including Kailikang, thereby displacing other forms of KLK1, including Kailikang.
We intend to conduct clinical trials to determine the pharmacokinetic and pharmacodynamic profile of Kailikang compared to DM199. While there have been numerous studies demonstrating the efficacy of Kailikang, we rely on the scientific and clinical knowledge and experience of other biotechnology and pharmaceutical companies and organizations in conducting those clinical studies. No assurance can be given that we will be able to reproduce results of previously conducted studies or displace other forms of KLK1 in the market.
We face the risk of product liability claims, which could exceed our insurance coverage and produce recalls, each of which could deplete our cash resources.
We are exposed to the risk of product liability claims alleging that use of our product candidates caused an injury or harm. These claims can arise at any point in the development, testing, manufacture, marketing, or sale of our product candidates and may be made directly by patients involved in clinical trials of our product candidates, by consumers or healthcare providers, or by individuals, organizations, or companies selling our products. Product liability claims can be expensive to defend, even if the product or product candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes increasingly expensive as a product candidate moves through the development pipeline to commercialization. To protect against potential product liability risks, we have AUD$20 million per occurrence and AUD$20 million aggregate clinical trial insurance for the REMEDY Phase 2 clinical trial in Australia and US$5.0 million product liability insurance coverage. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available to us at a cost acceptable to us or at all. We may choose or find it necessary under our collaboration agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for damages resulting from a product liability claim could exceed the amount of our coverage, require us to pay a substantial monetary award from our own cash resources and have a material adverse effect on our business, financial condition, and results of operations. Moreover, a product recall, if required, could generate substantial negative publicity about our products and business, inhibit or prevent commercialization of other products and product candidates, or negatively impact existing or future collaborations.
If we are unable to maintain product liability insurance required by our third parties, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
Some of our license, clinical trials and other agreements with third parties require, and in the future may require, us to maintain product liability insurance. If we cannot maintain acceptable amounts of coverage on commercially reasonable terms in accordance with the terms set forth in these agreements, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
A risk of product liability claims, and related negative publicity, is inherent in the development of human therapeutics and other products. Product liability insurance is expensive, its availability is limited, and it may not be offered on terms acceptable to us, or at all. The commercialization of our potential products could be inhibited or prevented by an inability to maintain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims. A product liability claim against us or the withdrawal of a product from the market could have a material adverse effect upon us and our financial condition.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We conduct certain research and development operations through our Australian wholly-owned subsidiary. If we lose our ability to operate in Australia, or if our subsidiary is unable to receive the research and development incentive payment allowed by Australian regulations, our business and results of operations could suffer.
In July 2016, we formed a wholly-owned Australian subsidiary, DiaMedica Australia Pty Ltd, to conduct various clinical activities for our product and development candidate in Australia. Due to the geographical distance and lack of employees currently in Australia, as well as our lack of experience operating in Australia, we may not be able to efficiently or successfully monitor, develop and commercialize our lead product in Australia, including conducting clinical trials. Furthermore, we have no assurance that the results of any clinical trials that we conduct for our product candidate in Australia will be accepted by the FDA or foreign regulatory authorities for development and commercialization approvals.
In addition, current Australian tax regulations provide for a refundable research and development incentive payment equal to 43.5% of qualified expenditures. We received incentive payments of approximately AUD$ 306,000 and AUD$ 777,000 during 2017 and 2018, respectively, for research expenditures made during 2016 and 2017. If our subsidiary loses its ability to operate in Australia, or if we are ineligible or unable to receive the research and development incentive payment, or the Australian government significantly reduces or eliminates the incentive program, our business and results of operation may be adversely affected.
Risks Related to Intellectual Property
If we are unable to adequately protect and enforce our intellectual property, our competitors may take advantage of our development efforts or acquired technology and compromise our prospects of marketing and selling our key product candidates.
We believe that patents and other proprietary rights are key to our business. Our policy is to file patent applications to protect technology, inventions, and improvements that may be important to the development of our business. We also rely upon trade secrets, know-how, continuing technological innovations, and licensing opportunities to develop and maintain our competitive position. We plan to enforce our issued patents and our rights to proprietary information and technology. We review third-party patents and patent applications, both to refine our own patent strategy and to identify useful licensing opportunities.
Our success depends, in part, on our ability to secure and protect our intellectual property rights and to operate without infringing on the proprietary rights of others or having third parties circumvent the rights owned or licensed by us. We have a number of patents, patent applications and rights to patents related to our compounds, product candidates and technology, but we cannot be certain that they will be enforceable or provide adequate protection or that pending patent applications will result in issued patents.
To the extent that development, manufacturing, and testing of our product candidates is performed by third party contractors, such work is performed pursuant to fee for service contracts. Under the contracts, all intellectual property, technology know-how, and trade secrets arising under such agreements are our exclusive property and must be kept confidential by the contractors. It is not possible for us to be certain that we have obtained from the contractors all necessary rights to such technologies. Disputes may arise as to the scope of the contract or possible breach of contract. No assurance can be given that our contracts would be enforceable or would be upheld by a court.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The patent positions of pharmaceutical and biotechnology firms, ourselves included, are uncertain and involve complex questions of law and fact for which important legal issues remain unresolved. Therefore, it is not clear whether our pending patent applications will result in the issuance of patents or whether we will develop additional proprietary products which are patentable. Part of our strategy is based on our ability to secure a patent position to protect our technology. There is no assurance that we will be successful in this approach and failure to secure patent protection may have a material adverse effect upon us and our financial condition. Also, we may fail in our attempt to commercialize products using currently patented or licensed technology without having to license additional patents. Moreover, it is not clear whether the patents issued or to be issued will provide us with any competitive advantages or if any such patents will be the target of challenges by third parties, whether the patents of others will interfere with our ability to market our products, or whether third parties will circumvent our patents by means of alternate processes. Furthermore, it is possible for others to develop products which have the same effect as our product candidates or technologies on an independent basis or to design around technologies patented by us. Patent applications relating to or affecting our business may have been filed by pharmaceutical or biotechnology companies or academic institutions. Such applications may conflict with our technologies or patent applications and such conflict could reduce the scope of patent protection which we could otherwise obtain or even lead to the rejection of our patent applications. There is no assurance that we can enter into licensing arrangements on commercially reasonable terms, or develop or obtain alternative technology in respect of, patents issued to third parties that incidentally cover our products or production technologies. Any inability to secure licenses or alternative technology could result in delays in the introduction of some of our product candidates or even lead to us being prevented from pursuing the development, manufacture, or sale of certain products. Moreover, we could potentially incur substantial legal costs in defending legal actions which allege patent infringement, or by initiating patent infringement suits against others. It is not possible for us to be certain that we are the creator of inventions covered by pending patent applications or that we were the first to invent or file patent applications for any such inventions. While we have used commercially reasonable efforts to obtain assignments of intellectual property from all individuals who may have created materials on our behalf (including with respect to inventions covered by our patent and pending patent applications), it is not possible for us to be certain that we have obtained all necessary rights to such materials. No assurance can be given that our patents, if issued, would be upheld by a court, or that a competitor’s technology or product would be found to infringe on our patents. Moreover, much of our technology know-how that is not patentable may constitute trade secrets. Therefore, we require our employees, consultants, advisors, and collaborators to enter into confidentiality agreements either as stand-alone agreements or as part of their consulting contracts. However, no assurance can be given that such agreements will provide meaningful protection of our trade secrets, know-how, or other proprietary information in the event of any unauthorized use or disclosure of confidential information. Also, while we have used commercially reasonable efforts to obtain executed copies of such agreements from all employees, consultants, advisors and collaborators, no assurance can be given that executed copies of all such agreements have been obtained.
We may require additional third-party licenses to effectively develop and manufacture our key products and are currently unable to predict the availability or cost of such licenses.
A substantial number of patents have already been issued to other biotechnology and pharmaceutical companies. To the extent that valid third-party patent rights cover our product candidates, we or our strategic collaborators would be required to seek licenses from the holders of these patents in order to manufacture, use, or sell these product candidates, and payments under them would reduce our profits from these product candidates. We are currently unable to predict the extent to which we may wish or be required to acquire rights under such patents, the availability and cost of acquiring such rights, and whether a license to such patents will be available on acceptable terms, or at all. There may be patents in the U.S. or in foreign countries or patents issued in the future that are unavailable to license on acceptable terms. Our inability to obtain such licenses may hinder or eliminate our ability to develop, manufacture and market our product candidates.
Changes in patent law and its interpretation could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology and pharmaceutical companies, our success is heavily dependent on intellectual property rights, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves technological and legal complexity, and obtaining and enforcing biopharmaceutical patents is costly, time consuming, and inherently uncertain. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office (“USPTO”), the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future. Changes in either the patent laws or interpretation of the patent laws in the United States or other countries could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our or our licensor’s patents or patent applications.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, and similar legislative, judicial, and administrative bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future.
Litigation regarding patents, patent applications, and other proprietary rights may be expensive, time consuming and cause delays in the development and manufacturing of our key product candidates.
Third parties may claim that we are using their proprietary information without authorization. Third parties may also have or obtain patents and may claim that technologies licensed to or used by us infringe their patents. If we are required to defend patent infringement actions brought by third parties, or if we sue to protect our own patent rights or otherwise to protect our proprietary information and to prevent its disclosure, we may be required to pay substantial litigation costs and managerial attention may be diverted from business operations even if the outcome is in our favor. In addition, any legal action that seeks damages or an injunction to stop us from carrying on our commercial activities relating to the affected technologies could subject us to monetary liability (including treble damages and attorneys’ fees if we are found to have willfully infringed) and require us or any third-party licensors to obtain a license to continue to use the affected technologies. We cannot predict whether we would prevail in any of these types of actions or that any required license would be available on commercially acceptable terms or at all. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources.
Competitors may infringe our patents or other intellectual property. If we were to initiate legal proceedings against a third party to enforce a patent covering our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Moreover, similar challenges may be made by third parties outside the context of litigation, e.g., via administrative proceedings such as post grant or inter partes review in the United States or via oppositions or other similar proceedings in other countries/regions.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Our defense of litigation, validity or enforceability, interference or derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation or such other proceedings could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are a party to a license agreement relating to an expression system and cell line for use in the production of DM199 or any human KLK1, and we may need to obtain additional licenses from others to advance our research and development activities or allow the commercialization of DM199 or any other product candidates we may identify and pursue. Future license agreements may impose, various development, diligence, commercialization, and other obligations on us. If any of our in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties main gain access to technologies that are material to our business, and we may be required to cease our development and commercialization of DM199 or other product candidates that we may identify or to seek alternative manufacturing methods. However, suitable alternatives may not be available or the development of suitable alternatives may result in a significant delay in our commercialization of DM199. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
|
● |
the scope of rights granted under the license agreement and other interpretation-related issues; |
|
● |
the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
|
● |
the sublicensing of patent and other rights under our collaborative development relationships; |
|
● |
our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
|
● |
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
|
● |
the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third parties to develop our products, we must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements, or other similar agreements with our collaborators, advisors, employees, and consultants prior to beginning research or disclosing proprietary information. These agreements typically restrict the ability of our collaborators, advisors, employees, and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. We also conduct joint research and development programs which may require us to share trade secrets under the terms of research and development collaboration or similar agreements. However, we cannot be certain that such agreements have been entered into with all relevant parties. Moreover, despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development, or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. Trade secrets can be difficult to protect. If the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secrets. A competitor’s discovery of our trade secrets may impair our competitive position and could have a material adverse effect on our business and financial condition.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Common Shares
Our common share price has been volatile in recent years and may continue to be volatile.
Our common shares currently trade in Canada on the TSX Venture Exchange under the trading symbol “DMA” and in the U.S. on the OTCQB Market under the trading symbol “DMCAF.” We have applied to list our common shares on Nasdaq under the trading symbol “DMAC.” A number of factors could influence the volatility in the trading price of our common shares, including changes in the economy or in the financial markets, industry related developments, and the impact of material events and changes in our operations. Our quarterly losses may vary because of expenses we incur related to future research including the timing of costs for manufacturing and initiating and completing preclinical and clinical trials. Each of these factors could lead to increased volatility in the market price of our common shares. In addition, the market prices of the securities of our competitors may also lead to fluctuations in the trading price of our common shares.
We have never paid dividends and do not expect to do so in the foreseeable future.
We have not declared or paid any cash dividends on our common shares to date. The payment of dividends in the future will be dependent on our earnings and financial condition and on such other factors as our Board of Directors considers appropriate. Unless and until we pay dividends, shareholders may not receive a return on their shares. There is no present intention by our Board of Directors to pay dividends on our common shares.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We may issue additional common shares resulting in share ownership dilution.
Future dilution may occur due to additional future equity financing events by us. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. In addition, if outstanding options, warrants, or deferred share units are exercised into our common shares, you will experience additional dilution.
It may be difficult for non-Canadian shareholders or other investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of Canada. Several of our directors and several of the experts we utilize are residents of Canada, and all or a substantial portion of their assets, and a portion of our assets, are located outside the United States. Consequently, it may be difficult for holders of our securities who reside in the United States to effect service within the United States upon those directors and the experts who are not residents of the United States. It may also be difficult for holders of our securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers, and experts under the United States federal securities laws. Our shareholders and other investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or such directors, officers, or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state or jurisdiction of the United States, or (ii) would enforce, in original actions, liabilities against us or such directors, officers, or experts predicated upon the United States federal securities laws or any securities or “blue sky” laws of any state or jurisdiction of the United States. In addition, the protections afforded by Canadian securities laws may not be available to our shareholders or other investors in the United States.
If there are substantial sales of our common shares, the market price of our common shares could decline.
Sales of substantial numbers of our common shares could cause a decline in the market price of our common shares. Any sales by existing shareholders or holders who exercise their warrants or stock options may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common shares.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline or increase in the market price of its securities or certain significant business transactions. We may become involved in this type of litigation in the future. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and our resources, which could harm our business.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common shares less attractive to our shareholders and other investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. We may remain an emerging growth company until the last day of the fiscal year following the fifth anniversary of our first sale of common shares pursuant to a registration statement under the Securities Act of 1933, as amended, or until such earlier time as we have more than $1.07 billion in annual revenue, the market value of our common shares held by non-affiliates is more than $700 million or we issue more than $1 billion of non-convertible debt over a three-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Our shareholders and other investors may find our common shares less attractive as a result of our reliance on these exemptions. If some of our shareholders or other investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and the trading price of our common shares may be more volatile.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised financial accounting standards. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we have determined to opt out of such extended transition period and, as a result, we will comply with new or revised financial accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised financial accounting standards is irrevocable.
Our shareholders and other investors may find our common shares less attractive as a result of our reliance on these exemptions. If some of our shareholders or other investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and the trading price of our common shares may be more volatile.
We will incur increased costs as a result of operating as a U.S. public reporting company, and our management is required to devote substantial time to new compliance initiatives.
As a U.S. public reporting company, we will incur, particularly after we are no longer an “emerging growth company,” significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and Nasdaq have imposed various requirements on U.S. public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We may have to hire additional accounting, finance, and other personnel in connection with our becoming a U.S. public reporting company, and our efforts to comply with the requirements of being a U.S. public reporting company, and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. These requirements increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
Our shareholder rights plan may delay or prevent an acquisition of us that shareholders may consider favorable or may prevent efforts by our shareholders to change our directors or our management, which could decrease the value of your shares.
Our shareholders approved the adoption of a shareholder rights plan agreement on December 21, 2017. The shareholder rights plan is designed to provide adequate time for our Board of Directors and shareholders to assess an unsolicited takeover bid for our company, to provide our Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The shareholder rights plan is set to expire at the close of our annual meeting of shareholders in 2020. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of our outstanding common shares without complying with the “permitted bid” provisions of the plan or without approval of our Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time. Under the plan, a “permitted bid” is a bid made to all holders of our common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
While we believe our rights plan enables our Board of Directors to help ensure that our shareholders are not deprived of the opportunity to realize the full and fair value of their investments, the rights plan may inhibit a change in control of our company by a third party in a transaction not approved by our Board of Directors. If a change in control is inhibited or delayed in this manner, it may adversely affect the market price of our common shares.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares. As a result of our limited administrative staffing levels, internal controls which rely on segregation of duties in many cases are not possible. Due to resource constraints and the present stage of our development, we do not have sufficient size and scale to warrant the hiring of additional staff to address this potential weakness at this time. To help mitigate the impact of this, we are highly reliant on the performance of compensating procedures and senior management’s review and approval. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with GAAP, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of shareholder or other investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our common shares.
Pursuant to Section 404 of the Sarbanes-Oxley Act, we will be required to furnish a report by our management on our internal control over financial reporting, and after we are no longer an emerging growth company, we will be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will have to engage in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. There is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Canadian laws differ from the laws in effect in the United States and may afford less protection to holders of our securities.
We are a Canadian corporation and are subject to the Canada Business Corporations Act and certain other applicable securities laws as a Canadian issuer, which laws may differ from those governing a company formed under the laws of a United States jurisdiction. The provisions under the Canada Business Corporations Act and other relevant laws may affect the rights of shareholders differently than those of a company governed by the laws of a United States jurisdiction, and may, together with our articles of continuance, have the effect of delaying, deferring or discouraging another party from acquiring control of our company by means of a tender offer, a proxy contest or otherwise, or may affect the price an acquiring party would be willing to offer in such an instance.
We are likely a “passive foreign investment company,” which may have adverse U.S. federal income tax consequences for U.S. shareholders.
Our U.S. shareholders and other U.S. investors should be aware that we believe we were classified as a passive foreign investment company, or PFIC, during the tax years ended December 31, 2017 and 2016, and based on current business plans and financial expectations, we believe that we likely will be a PFIC for the current tax year and may be a PFIC in future tax years. If we are a PFIC for any year during a U.S. shareholder’s holding period of our common shares, then such U.S. shareholder generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called “excess distribution” received on our common shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective “qualified electing fund” election, or QEF Election, or a “mark-to-market” election with respect to our shares. A U.S. shareholder who makes a QEF Election generally must report on a current basis its share of our net capital gain and ordinary earnings for any year in which we are a PFIC, whether or not we distribute any amounts to our shareholders. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the common shares over the shareholder’s adjusted tax basis therein. However, U.S. shareholders should be aware that there can be no assurance that we will satisfy the record keeping requirements that apply to a qualified electing fund, or that we will supply U.S. shareholders with information that such U.S. shareholders require to report under the QEF Election rules, in the event we are a PFIC and a U.S. shareholder wishes to make a QEF Election. Thus, U.S. shareholders may not be able to make a QEF Election with respect to their common shares. Each U.S. shareholder should consult its own tax advisors regarding the PFIC rules and the U.S. federal income tax consequences of the acquisition, ownership and disposition of our common shares.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 2. |
FINANCIAL INFORMATION. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is based upon accounting principles generally accepted in the United States of America and discusses the financial condition and results of operations for DiaMedica Therapeutics Inc. for the three months ended March 31, 2018 and 2017 and the years ended December 31, 2017 and 2016.
This discussion should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this registration statement. The following discussion contains forward-looking statements that involve numerous risks and uncertainties. Our actual results could differ materially from the forward-looking statements as a result of these risks and uncertainties. See “Special Note Regarding Forward-Looking Statements” for additional cautionary information.
Overview
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant proteins. Our goal is to use our patented and licensed technologies to establish our company as a leader in the development and commercialization of therapeutic treatments for novel recombinant proteins to treat neurological and kidney diseases. Our current primary focus is on AIS and CKD. We plan to advance DM199, our lead drug candidate, through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for AIS and CKD.
We have not generated any revenues from product sales. Since our inception, we have financed our operations from public and private sales of equity, the exercise of warrants and stock options, interest income on funds available for investment, and government grants and tax credits. We have incurred losses in each year since our inception. Our net losses were $650,000 and $1.5 million for the three months ended March 31, 2018 and 2017, respectively, and $4.3 million and $2.2 million for the years ended December 31, 2017 and 2016, respectively. As of March 31, 2018, we had an accumulated deficit of $40.9 million. Substantially all of our operating losses resulted from expenses incurred in connection with product candidate development programs, our research and development activities and general and administrative costs associated with our operations.
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. In the near term, we anticipate that our expenses will increase as we:
|
● |
advance the ongoing clinical development of DM199; |
|
● |
maintain, expand and protect our intellectual property portfolio; and |
|
● |
provide general and administrative support for our operations. |
To fund future operations we will need to raise additional capital. The amount and timing of future funding requirements will depend on many factors, including the timing and results of our ongoing development efforts, the potential expansion of our current development programs, potential new development programs and related general and administrative support. We anticipate that we will seek to fund our operations through public or private equity or debt financings or other sources, such as potential collaboration agreements. We cannot assure you that anticipated additional financing will be available to us on favorable terms, or at all. Although we have previously been successful in obtaining financing through our equity securities offerings, there can be no assurance that we will be able to do so in the future.
Financial Overview
Revenues
Since our inception, we have incurred losses while advancing the research and development of our therapeutic product candidates. We have not generated any revenues from product sales and do not expect to do so for a number of years. We may never generate revenues from our DM199 product candidate or any of our preclinical development programs, as we may never succeed in obtaining regulatory approval or commercializing any of these product candidates.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Research and Development Expenses
Research and development expenses consist primarily of fees paid to external service providers such as contract research organizations and contract manufacturing organizations related to clinical trials, contractual obligations for clinical development, clinical sites, laboratory testing, non-clinical trials, development of DM199 and the related manufacturing processes, salaries, benefits, share-based compensation and other personnel costs. We spent $791,000 and $1.1 million in research and development expenses for the quarters ended March 31, 2018 and 2017, respectively, and $3.2 million and $1.7 million for the years ended December 31, 2017 and 2016, respectively. Over the past approximately eight years, our research and development efforts have been primarily focused on DM199 for AIS and CKD.
At this time, due to the risks inherent in the clinical development process and the early stage of our product development programs, we are unable to estimate with any certainty the costs we will incur in the continued development of DM199 or any of our preclinical development programs. We expect that our research and development expenses may increase if we are successful in advancing DM199, or any of our preclinical programs into advanced stages of clinical development. The process of conducting clinical trials necessary to obtain regulatory approval and manufacturing scale-up to support expanded development and potential future commercialization is costly and time consuming. Any failure by us or delay in completing clinical trials, manufacturing scale-up or in obtaining regulatory approvals could lead to increased research and development expense and, in turn, have a material adverse effect on our results of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, including share-based compensation related to our executive, finance, business development and support functions. Other general and administrative expenses include rent and utilities, travel expenses and professional fees for auditing, tax and legal services. We expect that general and administrative expenses will increase in the future as we expand our operating activities. In addition, general and administrative expenses are expected to reflect increased costs associated with our anticipated U.S. public reporting company status and listing on the Nasdaq Stock Market. We anticipate incurring one-time costs associated with our anticipated Exchange Act registration and the listing of our common shares on Nasdaq of approximately $230,000 in 2018, consisting primarily of legal and accounting fees.
Other (Income) Expense
Other (income) expense consists primarily of governmental assistance – research incentives, change in the fair value of our warrants that are accounted for as liabilities, interest income, and foreign currency exchange gains and losses. In 2016, other expense was partially offset by the $250,000 gain recognized from the sale of a previous technology no longer being developed by the Company.
Critical Accounting Policies and Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and assumptions for the reported amounts of assets, liabilities, revenue, expenses and related disclosures. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material.
While our significant accounting policies are more fully described in Note 4 to our consolidated financial statements included elsewhere in this registration statement, we believe the following discussion addresses our most critical accounting policies, which are those that are most important to the portrayal of our financial condition and results of operations and require our most difficult, subjective and complex judgments.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Share-based Compensation
We account for all stock-based compensation awards using a fair value method. The cost of employee and non-employee services received in exchange for awards of equity instruments is measured and recognized based on the estimated grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the vesting period, which is considered to be the requisite service period. We record forfeitures in the periods in which they occur.
The fair value of stock-based awards is estimated using the Black-Scholes option pricing model. The determination of the fair value of stock-based awards is affected by our stock price, as well as assumptions regarding a number of complex and subjective variables. Risk free interest rates are based upon Canadian Government bond rates appropriate for the expected term of each award. Expected volatility rates are based on the on historical volatility equal to the expected life of the option. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past.
The assumptions used in calculating the fair value under the Black-Scholes option valuation model are set forth in the following table for options issued by the Company for the years ended December 31, 2017 and 2016:
|
2017 |
2016 |
|||||||
|
Common share fair value |
$0.26 – $0.42 |
$0.16 – 0.24 | ||||||
|
Risk-free interest rate |
1.1% | 0.8% | ||||||
|
Expected dividend yield |
0% | 0% | ||||||
|
Expected option life |
4.5 years |
4.6 years |
||||||
|
Expected stock price volatility |
84.7 – 156.8% | 92.0 – 185.1% | ||||||
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases. The guidance in ASU 2016-02 supersedes the lease recognition requirements in the Accounting Standards Codification Topic 840, Leases. ASU 2016-02 requires an entity to recognize assets and liabilities arising from a lease for both financing and operating leases, along with additional qualitative and quantitative disclosures. The new standard requires the immediate recognition of all excess tax benefits and deficiencies in the income statement and requires classification of excess tax benefits as an operating activity as opposed to a financing activity in the statements of cash flows. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, with early adoption permitted. Management is evaluating the impact of the new standard on the consolidated financial statements.
Results of Operations
Comparison of the Three Months Ended March 31, 2018 and 2017
The following table summarizes our results of operations for the three months ended March 31, 2018 and 2017. We did not have any revenue during those periods. The table below summarizes our expenses (in thousands):
|
Three Months Ended March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Research and development |
$ | 791 | $ | 1,072 | ||||
|
General and administrative |
515 | 283 | ||||||
|
Other (income) expense |
(657 | ) | 142 | |||||
Research and Development Expenses
Research and development expenses were $791,000 for the three months ended March 31, 2018 compared to $1.1 million for the same period in 2017, a decrease of $281,000. This decrease over the comparable prior year period was due primarily to lower levels of activity and study costs for the REMEDY Phase 2 stroke study as compared with the DM199 bridging study which was in progress during the comparable prior year period.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
General and Administrative Expenses
General and administrative expenses were $515,000 for the three months ended March 31, 2018 compared to $283,000 for the same period in 2017. General and administrative expenses increased due to greater usage of outside professional services and increased salaries, fees and short-term benefits due to the addition of staff. Share-based compensation expense increased related to the recognition of expense for awards granted during 2017.
Other (Income) Expense
Other (income) expense was $657,000 in income for the three months ended March 31, 2018 compared to $142,000 in expense for the same period in 2017. The increase in other income resulted primarily from the recognition of the research and development incentive from the Australian government for qualifying research work performed by DiaMedica Australia during 2017 and the first quarter of 2018.
Comparison of the Years Ended December 31, 2017 and 2016
The following table summarizes our results of operations for the years ended December 31, 2017 and 2016 (in thousands):
|
Year Ended December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Research and development |
$ | 3,206 | $ | 1,728 | ||||
|
General and administrative |
1,313 | 598 | ||||||
|
Other (income) expense |
(259 | ) | (106 | ) | ||||
Research and Development Expenses
Research and development expenses were $3.2 million for the year ended December 31, 2017 compared to $1.7 million for the year ended December 31, 2016, an increase of $1.5 million. The increase is primarily due to the costs incurred in conjunction with the advancement of the DM199 clinical trial program. Salaries, fees, and short-term benefits and share-based compensation also increased for the year ended December 31, 2017 over the comparable prior year period due to an increase in staff to support the clinical program.
General and Administrative Expenses
General and administrative expenses were $1.3 million for the year ended December 31, 2017 compared to $598,000 for the year ended December 31, 2016. General and administrative costs increased slightly due to an increase in outsourced services and salaries, fees, and short-term benefits, which were mainly due to an increase in staff. These increases were partially offset by decreased share-based compensation resulting from a reduction in the number of grants during 2017.
Other (Income) Expense
Other (income) expense was $259,000 in income for the year ended December 31, 2017 compared to $106,000 in income for 2016. Other income for 2017 increased due to the recognition of government assistance in the form of the research and development incentive tax credit received from Australia, related to qualifying clinical trial and other research expenses incurred by our Australian subsidiary.
Liquidity, Capital Resources and Going Concern
Since our inception, we have incurred losses while advancing the research and development of our therapeutic product candidates. We have not generated any revenues from product sales and do not expect to do so for a number of years. We do not know when, or if, we will generate any revenue from our product candidates. We do not expect to generate any revenue from sales of our product candidates unless and until we obtain regulatory approval. At the same time, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. In addition, we expect to incur additional costs associated with operating as a U.S. public reporting company. In addition, subject to obtaining regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need additional funding in connection with our continuing operations.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Since our inception, we have financed our operations from public and private sales of equity, the exercise of warrants and stock options, interest income on funds available for investment, and government grants and tax credits. We had cash totaling $6.7 million and $1.4 million and working capital of $6.6 million and $491,000 as of March 31, 2008 and December 31, 2017, respectively.
On March 29, 2018, we completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on March 19, 2020 and March 29, 2020 for tranche 1 and tranche 2, respectively. The warrants are subject to early expiration under certain conditions. In connection with the offering, we paid an aggregate cash fee of approximately $384,000 to brokers and issued an aggregate of approximately 1.6 million compensation options. Each compensation option entitles the holder to purchase one common share at $0.245, the offering price, for a period of two years from the closing of the offering, subject to acceleration on the same terms as the warrants issued to the investors.
The report of our independent registered public accounting firm on our December 31, 2017 audited consolidated financial statements includes an explanatory paragraph referring to our ability to continue as a going concern. Based upon our current operating plan, we expect to raise additional capital to be able to fund our planned operations beyond the next 12 months. This additional funding will be required to continue our research and development and other operating activities as we have not reached successful commercialization of our product candidates. These circumstances cast significant doubt as to our ability to continue as a going concern. Our consolidated financial statements have been prepared assuming that we will continue as a going concern. Our future operations are expected to continue to be dependent upon our ability to secure additional funds, negotiate license agreements with partners and/or generate product revenues in order to fully execute our business plan. There can be no assurance that we will be successful in commercializing our products, entering into strategic agreements with partners, raising additional capital on favorable terms or that these or other strategies will be sufficient to permit us to continue as a going concern.
The amount and timing of future funding requirements will depend on many factors, including the timing and results of our ongoing development efforts, the potential expansion of our current development programs, potential new development programs and related general and administrative support. We anticipate that we will seek to fund our operations through public or private equity or debt financings or other sources, such as potential collaboration agreements. We cannot assure you that anticipated additional financing will be available to us on favorable terms, or at all. Although we have previously been successful in obtaining financing through our equity securities offerings, there can be no assurance that we will be able to do so in the future. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. Debt financing, if available, may involve agreements that include conversion discounts or covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, or strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us.
Cash Flows
Operating Activities
Cash used in operating activities for the three months ended March 31, 2018 was $935,000 compared to $817,000 for the three months ended March 31, 2017. This increase relates primarily to the effects of the changes in operating assets and liabilities.
Cash used in operating activities for the year ended December 31, 2017 was $3.9 million, compared to $3.0 million for the year ended December 31, 2016, an increase of $0.9 million. This increase relates primarily to the increase in net loss, partially offset by an increase in non-cash charges for share-based compensation and the effects of changes in operating assets and liabilities.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Investing Activities
Investing activities consist primarily of purchases of property and equipment. Net cash used in investing activities was $32,000 for the three months ended March 31, 2018 compared to none for the three months ended March 31, 2017 and was $22,000 for the year ended December 31, 2017 compared to $7,000 for the year ended December 31, 2016.
Financing Activities
Financing activities consist primarily of net proceeds from the sale of common shares and warrants and proceeds from the exercise of stock options and warrants. Net cash provided by financing activities was $6.3 million for the three months ended March 31, 2018 compared to $7,000 for the three months ended March 31, 2017.
Net cash provided by financing activities was $3.5 million for the year ended December 31, 2017 compared to $4.6 million for the year ended December 31, 2016, a decrease of $1.1 million.
Cash flows from financing activities included net proceeds from the following private placements of our common shares and warrants to purchase common shares:
|
● |
On March 29, 2018, we completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on March 19, 2020 and March 29, 2020 for tranche 1 and tranche 2, respectively. The warrants are subject to early expiration under certain conditions. In connection with the offering, we paid an aggregate cash fee of approximately $384,000 to brokers and issued an aggregate of approximately 1.6 million compensation options. Each compensation option entitles the holder to purchase one common share at $0.245, the offering price, for a period of two years from the closing of the offering, subject to acceleration on the same terms as the warrants issued to the investors. |
|
● |
On December 18, 2017, we completed a non-brokered private placement of 3,624,408 units at a price of $0.26 per unit for aggregate gross proceeds of approximately $944,000. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on December 19, 2019, subject to early expiration under certain conditions. |
|
● |
On April 17, 2017, we completed a non-brokered private placement of 10,526,315 units at a price of $0.19 per unit for aggregate proceeds of approximately $2,000,000. Each unit consists of one common share and one-half common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.23 at any time prior to expiration on April 17, 2019. The warrant expiration date can be accelerated at our option in the event that the volume-weighted average trading price of our common shares exceeds $0.30 per common share for any 10 consecutive trading days. |
|
● |
On September 8, 2016, we completed the second tranche of a non-brokered private placement of 15,000,000 common shares at a price of $0.20 per share for aggregate gross proceeds of $3,000,000. |
|
● |
On August 22, 2016, we completed the first tranche of a non-brokered private placement of 5,000,000 common shares at a price of $0.20 per share for aggregate gross proceeds of $1,000,000. |
|
● |
On February 25, 2016, we completed the second tranche of a non-brokered private placement of 875,000 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $101,710. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitled the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiration of two years from the closing date. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
● |
On February 18, 2016, we completed the first tranche of a non-brokered private placement of 3,812,500 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $445,544. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitled the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiration of two years from the closing date. |
While our rate of future negative cash flow per month will vary due to the timing of expenses incurred, at the current rate of negative cash flow per month we believe that our current cash will enable us to complete our currently ongoing Phase 2 trial in patients with AIS and initiate a Phase 1b trial in patients with CKD. Our future cash requirements will increase if we decide to expand our research and development efforts beyond the currently planned development of DM199.
Commitments and Contingencies
In the normal course of business, we incur obligations to make future payments as we execute our business plan. As of March 31, 2018, we had outstanding commitments, including research and development contracts and other commitments, that are known and committed of approximately $2.1 million over the next 12 months and approximately $900,000 in the following 12 months. These contracts relate to preclinical, clinical, and development activities, including the clinical research organization conducting the Phase II clinical trial for DM199 related to AIS. These commitments are subject to significant change and the ultimate amounts due may be materially different as these obligations are affected by, among other factors, the number and pace of patients enrolled, the number of clinical study sites, amount of time to complete study enrollments and the time required to finalize the analysis and reporting of study results. These commitments are generally cancelable upon 30 days’ notice, with our obligation then limited to costs incurred up to that date. As of March 31, 2018, we had future operating lease commitments totaling approximately $275,000 over the remainder of the lease, of which $62,000 is due over the next 12 months.
We have entered into a research, development and license agreement whereby we receive research services and rights to proprietary technologies. Milestone and royalty payments that may become due under this agreement are dependent upon, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain. As of March 31, 2018, two milestones remain which include $185,000 due upon the initiation of dosing in our first Phase III trial and $185,000 upon our first regulatory approval for commercial sale. Following the launch of our first product, we will also incur a royalty of less than 1% on net sales.
Off-Balance Sheet Arrangements
During 2017 and 2016 and the three months ended March 31, 2018, we did not have any off-balance sheet arrangements (as defined by applicable SEC regulations) that are reasonably likely to have a current or future material effect on our financial condition, results of operations, liquidity, capital expenditures or capital resources.
Internal Control Over Financial Reporting
Pursuant to Section 404(a) of the Sarbanes-Oxley Act, commencing the year following our first annual report required to be filed with the SEC, our management will be required to report on the effectiveness of our internal control over financial reporting. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. To comply with the requirements of being a reporting company under the Exchange Act, we may need to upgrade our systems, including information technology, implement additional financial and management controls, reporting systems and procedures and hire additional accounting and finance staff.
|
ITEM 3. |
PROPERTIES. |
Our principal executive offices, together with our research and development operations, are at the office of our wholly owned subsidiary, DiaMedica USA Inc., located at 2 Carlson Parkway, Suite 260, Minneapolis, Minnesota, USA 55447. We lease these premises, which consist of approximately 3,800 square feet, pursuant to a lease that expires in August 2022. Our registered Canadian office is located at 301-1665 Ellis Street, Kelowna, British Columbia, V1Y 2B3. The registered office for our Australian subsidiary is located at Level 9, Bourke Street, Melbourne, Victoria, Australia. We believe that our facilities are adequate for our current needs and that suitable additional space will be available as and when needed on acceptable terms.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 4. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT. |
The following table sets forth information known to us with respect to the beneficial ownership of our common shares as of August 20, 2018 for:
|
● |
each person known by us to beneficially own more than five percent of the outstanding shares of our common shares; |
|
● |
each of our directors; |
|
● |
each of the executive officers named in the Summary Compensation Table included later in this registration statement under “Executive Compensation;” and |
|
● |
all of our current directors and executive officers as a group. |
The number of shares beneficially owned by a person includes shares subject to options or warrants held by that person that are currently exercisable or that become exercisable within 60 days of August 20, 2018 and the issuance of common shares upon the vesting of deferred stock units (“DSU”). Percentage calculations assume, for each person and group, that all shares that may be acquired by such person or group pursuant to options currently exercisable or that become exercisable within 60 days of August 20, 2018 are outstanding for the purpose of computing the percentage of common shares owned by such person or group. However, such unissued shares of common shares described above are not deemed to be outstanding for calculating the percentage of common shares owned by any other person.
Except as otherwise indicated, the persons in the table below have sole voting and dispositive power with respect to all common shares shown as beneficially owned by them, subject to community property laws where applicable and subject to the information contained in the notes to the table.
|
Title of Class |
Name and Address of Beneficial Owner(1) |
Amount and Nature of Beneficial Ownership(2) |
Percent of Class |
|||||||
|
Directors and Officers: |
||||||||||
|
Common Shares |
Richard Pilnik |
1,245,100 | * | |||||||
|
Common Shares |
Michael Giuffre, M.D. |
3,778,994 | (3) | 2.4 | % | |||||
|
Common Shares |
James Parsons |
407,000 | * | |||||||
|
Common Shares |
Zhenyu Xiao, Ph.D. |
20,128,667 | (4) | 12.8 | % | |||||
|
Common Shares |
Rick Pauls |
3,336,252 | 2.1 | % | ||||||
|
Common Shares |
Todd Verdoorn |
724,583 | * | |||||||
|
Common Shares |
All current directors and executive officers as a group (8 persons) |
29,849,296 | 18.3 | % | ||||||
|
Significant Beneficial Owners: |
||||||||||
|
Common Shares |
Hermeda Industrial Co., Limited Level 54 Hopewell Centre 183 Queensroad East Hong Kong |
20,000,000 | (4) | 12.8 | % | |||||
|
Common Shares |
CentreStone Ventures, LP 4-1250 Waverley Street Winnipeg, Manitoba R3T 6C6 Canada |
14,118,335 | (5) | 9.0 | % | |||||
|
Common Shares |
Nancy Chang 101 Westcott, Unit 603 Houston, TX 77007 |
13,207,894 | (6) | 8.4 | % | |||||
* Represents beneficial ownership of less than one percent.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
(1) |
The business address for each of the directors and officers is c/o DiaMedica Therapeutics Inc., 2 Carlson Parkway, Suite 260, Minneapolis, MN 55447. |
|
(2) |
Includes for the persons listed below the following common shares subject to options and warrants held by such persons that are currently exercisable or become exercisable within 60 days of August 20, 2018 and common shares issuable upon the vesting of DSUs within 60 days of August 20, 2018: |
|
Name |
Common Shares Underlying Stock Options |
Common Shares Underlying Warrants |
Common Shares Underlying DSUs |
|||||||||
|
Directors |
||||||||||||
|
Richard Pilnik |
893,333 | — | 151,767 | |||||||||
|
Michael Giuffre, M.D. |
455,000 | 224,490 | 82,924 | |||||||||
|
James Parsons |
330,000 | — | 77,000 | |||||||||
|
Zhenyu Xiao, Ph.D. |
51,667 | — | 77,000 | |||||||||
|
Named Executive Officers |
||||||||||||
|
Rick Pauls |
2,799,167 | 41,000 | 34,985 | |||||||||
|
Todd Verdoorn |
704,583 | — | — | |||||||||
|
All current directors and executive officers as a group (7 persons) |
5,401,250 | 285,890 | 423,676 | |||||||||
|
(3) |
Includes: (i) 103,300 common shares held by 424822 Alberta Ltd, Michael Giuffre, M.D. has sole voting and dispositive power over the common shares held by 424822 Alberta Ltd., (ii) 729,964 common shares Dr. Giuffre and his wife hold jointly, (iii) 1,083,716 common shares held by Dr. Giuffre’s sons and daughters, (iv) 421,400 common shares held by Dr. Giuffre’s wife and (v) 678,200 common shares held directly by Dr. Giuffre. |
|
(4) |
Includes 20,000,000 common shares held by Hermeda Industrial Co., Limited. Zhenyu Xiao, Ph.D. is the Managing Director of Hermeda Industrial Co., Limited and has sole voting and dispositive power over the common shares held by Hermeda Industrial Co., Limited. |
|
(5) |
Albert D. Friesen, the managing director of CentreStone Ventures, Inc., has sole voting and dispositive power over the common shares held by CentreStone Ventures, LP. |
|
(6) |
Includes 789,390 shares held by the Chang Family Foundation. Nancy Chang has sole voting and dispositive power over the common shares held by Chang Family Foundation. Also includes 50,000 common shares subject to an option that is currently exercisable or becomes exercisable within 60 days of August 20, 2018. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 5. |
DIRECTORS AND EXECUTIVE OFFICERS. |
The following table sets forth information as of August 20, 2018 regarding each of our current executive officers and directors:
|
Name |
Age |
Positions |
||
|
Rick Pauls |
47 |
President and Chief Executive Officer, Director |
||
|
Scott Kellen |
53 |
Chief Financial Officer and Secretary |
||
|
Todd Verdoorn, Ph.D. |
57 |
Chief Scientific Officer |
||
|
Harry Alcorn, Pharm.D. |
62 |
Chief Medical Officer and Vice President of Clinical Affairs |
||
|
Richard Pilnik(1)(2)(3) |
61 |
Chairman of the Board |
||
|
Michael Giuffre, M.D.(1)(2)(3) |
63 |
Director |
||
|
James Parsons(1)(2)(3) |
52 |
Director |
||
|
Zhenyu Xiao, Ph.D. |
44 |
Director |
(1) Member of the Audit Committee.
(2) Member of the Compensation Committee.
(3) Member of the Governance and Nominating Committee.
The present principal occupations and recent employment history of each of our executive officers and directors are set forth below. Pursuant to the terms of our articles of continuance and Canada law, at least 25% of our directors must be resident Canadians:
Executive Officers
Rick Pauls was appointed our President and Chief Executive Officer in January 2010. Mr. Pauls has served as a member of our Board of Directors since April 2005 and the Chairman of the Board from April 2008 to July 2014. Prior to joining DiaMedica, Mr. Pauls was the Co-Founder and Managing Director of CentreStone Ventures Inc., a life sciences venture capital fund, from February 2002 until January 2010. Mr. Pauls was an analyst for Centara Corporation, another early stage venture capital fund, from January 2000 until January 2002. From June 1997 until November 1999, Mr. Pauls worked for General Motors Acceptation Corporation specializing in asset-backed securitization and structured finance. Mr. Pauls previously served as an independent member of the board of directors of LED Medical Diagnostics, Inc. Mr. Pauls received his Bachelor of Arts in Economics from the University of Manitoba and his M.B.A. in Finance from the University of North Dakota.
We believe that Mr. Pauls’s experience in the biopharmaceutical industry as an executive and investor and his extensive knowledge of all aspects of our company, business, industry, and day-to-day operations as a result of his role as our President and Chief Executive Officer enable him to make valuable contributions to our Board of Directors. In addition, as a result of his role as President and Chief Executive Officer, Mr. Pauls provides unique insight into our future strategies, opportunities and challenges, and serves as the unifying element between the leadership and strategic direction provided by our Board of Directors and the implementation of our business strategies by management.
Scott Kellen was appointed our Chief Financial Officer and Secretary in April 2018. Prior to joining DiaMedica, Mr. Kellen served as Vice President and Chief Financial Officer of Sun BioPharma, Inc., a publicly-traded clinical stage drug development company, from October 2015 until April 2018. From February 2010 to September 2015, Mr. Kellen served as Chief Financial Officer and Secretary of Kips Bay Medical, Inc., a publicly-traded medical device company, and became Chief Operating Officer of Kips Bay in March 2012. From November 2007 to May 2009, Mr. Kellen served as Finance Director of Transoma Medical, Inc. From 2005 to October 2007, Mr. Kellen served as Corporate Controller of ev3 Inc. From March 2003 to April 2005, Mr. Kellen served as Senior Manager, Audit and Advisory Services of Deloitte & Touche, LLP. Altogether, Mr. Kellen has spent more than 25 years in the life sciences industry, focusing on publicly traded early stage and growth companies. Mr. Kellen has a Bachelor of Science degree in Business Administration from the University of South Dakota and is a Certified Public Accountant (inactive).
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Todd Verdoorn, Ph.D. was appointed our Chief Scientific Officer in May 2016. From January 2016 to April 2016, Dr. Verdoorn served as our Vice President, Neuroscience. Prior to joining DiaMedica, Dr. Verdoorn served as Chief Scientist at Intuitive Quantitation, LLC, a company that provides strategic and tactical leadership for companies creating new treatments, from May 2013 to December 2016. From September 2011 to May 2013, Dr. Verdoorn served as Vice President, Neurobiology at NeuroTherapeutics Pharma, Inc., a company that develops and markets therapeutics. From January 2008 to August 2011, Dr. Verdoorn served as Chief Scientist for Orasi Medical, Inc., a medical device company. From June 2007 to January 2008, Dr. Verdoorn served as Chief Scientific Officer for Smart Bioscience SAS, a company that discovers and develops small-molecule therapeutics. Prior to joining Smart Bioscience, Dr. Verdoorn served as Chief Scientific Officer at Algos Preclinical Services, Inc., a research and consulting company, from January 2003 to June 2007. Dr. Verdoorn has more than 26 years of experience working with both public and private companies to develop new treatments for neurological diseases, including five years working with Bristol-Myers Squibb’s stroke group. Dr. Verdoorn has a Bachelor of Arts degree in Chemistry from Central College and he earned his Ph.D. in Neurobiology from the University of North Carolina, conducting his post-doctoral research at the Max Planck Institute with Nobel Laureate Dr. Bert Sakmann and served as Associate Professor of Pharmacology at Vanderbilt University School of Medicine.
Harry Alcorn Jr. Pharm.D. was appointed our Vice President of Medical Affairs in August 2018. Prior to joining DiaMedica, Dr. Alcorn served as Chief Scientific Officer at DaVita Clinical Research (“DCR”), a company that provides clinical research services for Pharmaceutical and Biotech companies, from October 1997 to June 2018. While at DCR, Dr. Alcorn was responsible for clinical research operations, including the formation and management of the early clinical and late phase research services. Dr. Alcorn also founded the U.S. Renal Network, the first network of Phase 1 renal research sites in the United States. Dr. Alcorn developed DCR's site management organization for clinical trials. Dr. Alcorn also served as an Executive Director, a Pharmacist and an Investigator at DCR. During this time, from Jan 2013 to December 2014, he also served on the Board of Directors for the Association of Clinical Pharmacology Units, an association of Phase I clinical trial sites. Dr. Alcorn has over 30 years of clinical research experience working with Biotech and Pharmaceutical companies, both public and private, in conducting research in renal, hepatic and cardiovascular disease. Dr. Alcorn has written and consulted on the development of several protocols and has served as Principal Investigator or Sub Investigator in numerous studies and, for several of these studies, presented study design and results to the FDA. Currently he holds clinical faculty appointments with the University of Minnesota, Creighton University, University of Nebraska Medical Center, Virginia Commonwealth and the University of Colorado, Denver. Dr. Alcorn graduated from Creighton University with a Bachelor of Pharmacy and went on to earn his Doctor of Pharmacy degree from University of Nebraska Medical Center.
Non-Employee Directors
Richard Pilnik has served as a member of our board of directors since May 2009. Mr. Pilnik serves as our Chairman of the Board. Mr. Pilnik has served as the President and member of the board of directors of Vigor Medical Services, Inc., a medical device company, since May 2017. From December 2015 to November 2017, Mr. Pilnik served as a member of the board of directors of Chiltern International Limited, a private leading mid-tier Clinical Research Organization, and was Chairman of the Board from April 2016 to November 2017. Mr. Pilnik has a 30-year career in healthcare at Eli Lilly and Company, a pharmaceutical company, and Quintiles Transnational Corp., a global pioneer in pharmaceutical services. From April 2009 to June 2014, Mr. Pilnik served as Executive Vice President and President of Quintiles Commercial Solutions, an outsourcing business to over 70 pharma and biotech companies. Prior to that, he spent 25 years at Eli Lilly and Company where he held several leadership positions, most recently as Group Vice President and Chief Marketing Officer from May 2006 to July 2008. Mr. Pilnik was directly responsible for commercial strategy, market research, new product planning and the medical marketing interaction. From December 2000 to May 2006, Mr. Pilnik served as President of Eli Lilly Europe, Middle East and Africa and the Commonwealth of Independent States, a regional organization of former Soviet Republics, and oversaw 50 countries and positioned Eli Lilly as the fastest growing pharmaceutical company in the region. Mr. Pilnik also held several marketing and sales management positions in the United States, Europe and Latin America. Mr. Pilnik currently serves on the board of directors of Vigor Medical Systems, Inc., NuSirt, an early-stage biopharma, and the Duke University Fuqua School of Business. Mr. Pilnik previously served on the board of directors of Elan Pharmaceuticals, Chiltern International, the largest mid-size Clinical Research Organization, and Certara, L.P., a private biotech company focused on drug development modeling and biosimulation. Mr. Pilnik holds a Bachelor of Arts in Economics from Duke University and an MBA from the Kellogg School of Management at Northwestern University.
We believe that Mr. Pilnik’s deep experience in the industry and his history and knowledge of our company enable him to make valuable contributions to our Board of Directors.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Michael Giuffre, M.D. has served as a member of our Board of Directors since August 2010. Since July 2009, Dr. Giuffre has served as a Clinical Professor of Cardiac Sciences and Pediatrics at the University of Calgary and has had an extensive portfolio of clinical practice, cardiovascular research and university teaching. Dr. Giuffre is actively involved in health care delivery, medical leadership and in the biotechnology business sector. Since 2012, Dr. Giuffre has served as the Chief Scientific Officer and a member of the board of directors of FoodChek Systems Inc. and in November 2017, he became Chairman of the Board. Dr. Giuffre also serves as President of FoodChek Laboratories Inc. Dr. Giuffre previously served on the board of directors of the Canadian Medical Association (CMA), Unicef Canada, the Alberta Medical Association (AMA), Can-Cal Resources Ltd, Vacci-Test Corporation, IC2E International Inc. and MedMira Inc. Dr. Giuffre has received a Certified and Registered Appointment and a Distinguished Fellow appointment by the American Academy of Cardiology (FACC). In 2005, he was awarded Physician of the Year by the Calgary Medical Society and in 2017 was “Mentor of the Year” for the Royal College of Physicians and Surgeons of Canada. Dr. Giuffre was also a former President of the AMA and the Calgary and Area Physicians Association and also a past representative to the board of the Calgary Health Region. Dr. Giuffre holds a Bachelor of Science in cellular and microbial biology, a Ph.D. candidacy in molecular virology, an M.D. and an M.B.A. He is Canadian Royal College board certified in specialties that include Pediatrics and Pediatric Cardiology and has a subspecialty in Pediatric Cardiac Electrophysiology. Dr. Giuffre is a member of the board of directors of Avenue Living, a private real estate company in Calgary, Alberta, Canada and its affiliates, Avondale Real Estate Capital Ltd. and AgriSelect Land Capital, Ltd., both private real estate companies in Calgary, Alberta Canada. Dr. Giuffre is a resident of Canada.
We believe that Dr. Giuffre’s medical experience, including as a practicing physician and professor, enable him to make valuable contributions to our Board of Directors.
James Parsons has served as a member of our Board of Directors since October 2015. Previously, Mr. Parsons served as our Vice President of Finance from October 2010 until May 2014. Since August 2011, Mr. Parsons has served as Chief Financial Officer and Corporate Secretary of Trillium Therapeutics Inc., a Nasdaq-listed immuno-oncology company. Mr. Parsons serves as a member of the board of directors and audit committee chair of Sernova Corp., which is listed on the TSX Venture Exchange. Mr. Parsons has been a Chief Financial Officer in the life sciences industry since 2000 with experience in therapeutics, diagnostics and devices. Mr. Parsons has a Master of Accounting degree from the University of Waterloo and is a Chartered Professional Accountant and Chartered Accountant. Mr. Parsons is a resident of Canada.
We believe that Mr. Parsons’ financial experience, including his history and knowledge of our company, enable him to make valuable contributions to our Board of Directors.
Zhenyu Xiao, Ph.D. has served as a member of our Board of Directors since November 2016. Dr. Xiao was elected to our Board of Directors in connection with the equity investment by Hermeda Industrial Co., Limited and is its designee to the Board of Directors under an investment agreement which is described in more detail under “Item 7. Certain Relationships and Related Transactions, and Director Independence.” Dr. Xiao has been the Chief Executive Officer of Hermed Equity Investment Management (Shanghai) Co., Ltd., a private equity fund. From June 2008 to November 2014, Dr. Xiao was the Associate General Manager of Shanghai Fosun Pharmaceutical Group Co Ltd., a pharmaceutical manufacturing company, where he was the deputy chief of the IPO team for the Fosun Pharma Listing in Hong Kong Exchange and the deputy director of Fosun Pharmaceutical Technological Center in charge of evaluating new technology and R&D and investment. Dr. Xiao has a Ph.D. degree in Pharmacology and conducted his postdoctoral research at University of Rochester (NY), co-founding a pharmaceutical company with Dr. Paul Okunieff and winning Small Business Technology Transfer support, a U.S. Small Business Administration program to facilitate joint venture opportunities between small businesses and non-profit research institutions.
We believe that Dr. Xiao’s experience in the industry, including as an investor, enable him to make valuable contributions to our Board of Directors.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 6. |
EXECUTIVE COMPENSATION. |
Executive Compensation Overview
The Compensation Committee of our Board of Directors administers our executive compensation programs on behalf of our Board of Directors. The Compensation Committee has a charter that will be reviewed and updated annually, or as may be warranted from time to time. The current members of the Compensation Committee are Michael Giuffre, M.D. (Chair), James Parsons and Richard Pilnik.
This section addresses the compensation of our President and Chief Executive Officer and the only other executive officer of the Company as of December 31, 2017:
|
● |
Rick Pauls, our President and Chief Executive Officer; and |
|
● |
Todd Verdoorn, Ph.D., our Chief Scientific Officer. |
The above executive officers are collectively referred to as the named executive officers.
The elements of the compensation program for our named executive officers include:
|
● |
base salary; |
|
● |
long-term equity-based incentive compensation; |
|
● |
annual incentive compensation; and |
|
● |
other compensation, including certain health, welfare and retirement benefits and, when determined necessary, limited perquisites. |
The named executive officers also have termination and change in control benefits in their respective employment agreements.
When reading this Executive Compensation Overview, please note that we are an emerging growth company under the JOBS Act and are not required to provide a “Compensation Discussion and Analysis” of the type required by Item 402 of Regulation S-K. This Executive Compensation Overview is intended to supplement the SEC-required disclosure, which is included below this section, and it is not a Compensation Discussion and Analysis.
Base Salary
We provide a base salary for our named executive officers, which, unlike some of the other elements of our executive compensation program, is not subject to company or individual performance risk. We recognize the need for most executives to receive at least a portion of their total compensation in the form of a guaranteed base salary that is paid in cash regularly throughout the year. The base salaries set for our named executive officers are intended to provide a steady income regardless of share price performance, allowing executives to focus on both near-term and long-term goals and objectives without undue reliance on short term share price performance or market fluctuations.
We initially fix base salaries for our executives at a level that we believe enables us to hire and retain them in a competitive environment and to reward satisfactory individual performance and a satisfactory level of contribution to our overall business objectives. The Compensation Committee reviews and approves any increases in base salaries for our named executive officers.
The Compensation Committee has the authority to engage the services of outside experts and advisors as it deems necessary or appropriate to carry out its duties and responsibilities, and prior to doing so, assesses the independence of such experts and advisors from management.
Our Chief Executive Officer assists the Compensation Committee in gathering compensation related data regarding our executive officers and making recommendations to the Compensation Committee regarding the form and amount of compensation to be paid to each executive officer. In addition, the Compensation Committee has retained 21-Group, a compensation consultant, to assist in the design and review of certain aspects of our executive compensation program. The 21-Group does not provide any services to our company other than those for which it has been retained by the Compensation Committee. The Compensation Committee has assessed the independence of the 21-Group pursuant to Securities and Exchange Commission and Nasdaq rules and has concluded that the work of the 21-Group does not raise any conflicts of interest.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
In making final decisions regarding compensation to be paid to our executive officers, the Compensation Committee considers the recommendations of our Chief Executive Officer, the data compiled and recommendations of the 21-Group, as well as its own views as to the form and amount of compensation to be paid, the general performance of our company and the individual officers, the performance of our common share price and other factors that may be relevant. Final deliberations and decisions by the Compensation Committee regarding the form and amount of compensation to be paid to our executive officers, including our Chief Executive Officer, are made by the Compensation Committee, without the presence of the Chief Executive Officer or any other executive officer of our company.
Annualized base salary rates for each of our named executive officers for fiscal 2017 and the current fiscal 2018 are as follows:
|
Name |
Fiscal 2017 |
Fiscal 2018 |
% Change From Fiscal 2017 |
|||||||||
|
Rick Pauls |
$ | 280,000 | $ | 345,000 | 23 | |||||||
|
Todd Verdoorn |
200,000 | 240,000 | 20 | |||||||||
Long-Term Equity-Based Incentive Compensation
The long-term equity-based incentive compensation component consists of stock options granted under the DiaMedica Therapeutics Inc. Stock Option Plan, or Stock Option Plan, which generally vest quarterly over a three-year period and deferred share units, or DSUs, granted under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan, or DSU Plan. These plans are designed to give each option and DSU holder an interest in preserving and maximizing shareholder value in the long term, to enable us to attract and retain individuals with experience and ability, and to reward individuals for current performance and expected future performance. Long-term equity-based incentives are intended to comprise a significant portion of each executive’s compensation package, consistent with our executive compensation objective to align the interests of our executives with the interests of our shareholders.
The Compensation Committee uses stock options as a portion of the long-term equity based incentive compensation component since the Compensation Committee believes that options effectively incentivize executives to maximize company performance, as the value of awards is directly tied to an appreciation in the value of our common shares. Stock options also provide an effective retention mechanism because of vesting provisions. An important objective of our long-term equity-based incentive program is to strengthen the relationship between the long-term value of our common shares and the potential financial gain for our executives. Stock options provide recipients with the opportunity to purchase our common shares at a price fixed on the grant date regardless of future market price. Because stock options become valuable only if the share price increases above the exercise price and the option holder remains employed during the period required for the option to vest, they provide an incentive for an executive to remain employed. In addition, stock options link a portion of an executive’s compensation to the interests of our shareholders by providing an incentive to achieve corporate goals and increase the market price of our common shares over the vesting period.
The Compensation Committee previously used DSUs as a portion of the long-term equity-based incentive compensation component in order to provide an alternative form of compensation to satisfy annual and special bonuses payable to our executive officers. The DSU Plan provided that the Board of Directors may, from time to time, issue DSUs to our executive officers at the time of declaring or awarding any bonuses. The number of DSUs granted was determined by dividing the applicable bonus amount by the fair market value of our common shares as of the last trading day before the award date as calculated. No DSUs were granted during 2018 or 2017.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The table below sets forth the stock options that we granted to our named executive officers in 2017 and to date in 2018:
|
Name |
Grant Date |
Number of Shares Underlying Options |
Exercise Price CAD$ |
|||||||
|
Rick Pauls |
06/19/17 |
850,000 | 0.32 | |||||||
|
04/17/18 |
670,000 | 0.56 | ||||||||
|
Todd Verdoorn |
06/19/17 |
500,000 | 0.32 | |||||||
|
04/17/18 |
435,500 | 0.56 | ||||||||
Annual Incentive Compensation
In addition to base salary and long-term equity based incentive compensation, we provide our named executive officers the opportunity to earn annual incentive compensation based on the achievement of certain company and individual related performance goals. Our annual bonus program directly aligns the interests of our executive officers and shareholders by providing an incentive for the achievement of key corporate and individual performance measures that are critical to the success of our company and linking a significant portion of each executive’s annual compensation to the achievement of such measures.
All Other Compensation
It is generally our policy not to extend significant perquisites to our executives that are not available to our employees generally. Our executives receive benefits that are also received by our other employees, including participation in the DiaMedica USA, Inc. 401(k) Plan and health, dental, disability and life insurance benefits.
Employment Agreements
We expect to enter into an employment agreement with each of our executive officers prior to the effective date of this registration statement. These executive employment agreements will provide for an annual base salary, which will be subject to periodic reviews, incentive compensation, and equity-based compensation. The employment agreement also will contain severance protection as described in more detail below and standard confidentiality, non-competition, non-solicitation and assignment of intellectual property provisions.
Change in Control and Post-Termination Severance Arrangements
Change in Control Arrangements
To encourage continuity, stability and retention when considering the potential disruptive impact of an actual or potential corporate transaction, we have established change in control arrangements, including provisions in our Stock Option Plan and written employment agreements that we intend to enter into with our executives. These arrangements are designed to incentivize our executives to remain with our company in the event of a change in control or potential change in control.
Under the terms of the employment agreements that we intend to enter into with our executives and consistent with the terms of their offer letters, in the event of a voluntary resignation by the executive or a termination by the Company without cause of the employment of Mr. Pauls or Mr. Verdoorn within 60 days of a change in control, the executive will be entitled to receive 18 months of base pay plus applicable bonus in the case of Mr. Pauls and nine months of base salary in the event of Mr. Verdoorn. In addition, their stock options granted under the Stock Option Plan will immediately accelerate in full under such termination.
We believe these change in control arrangements are an important part of our executive compensation program in part because they mitigate some of the risk for executives working in a smaller company where there is a meaningful risk that the company may be acquired. Change in control benefits are intended to attract and retain qualified executives who, absent these arrangements and in anticipation of a possible change in control of our company, might consider seeking employment alternatives to be less risky than remaining with our company through the transaction. We believe that the form and amount of these change in control benefits are fair and reasonable to both our company and our executives. The Compensation Committee intends to review our change in control arrangements periodically to ensure that they remain necessary and appropriate.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Other Severance Arrangements
Under the terms of the executive employment agreement that we intend to enter into with our executives and consistent with the terms of their offer letters, in the event of a termination by the Company without cause of the employment of Mr. Pauls or Mr. Verdoorn, the executive will be entitled to receive 12 months of base pay plus applicable bonus in the case of Mr. Pauls and six months of base salary in the event of Mr. Verdoorn. In addition, Mr. Pauls’s stock options granted under the Stock Option Plan will continue to vest for six months and vested options will remain exercisable for six months from the date of termination. We believe that the form and amount of these severance benefits are fair and reasonable to both our company and our executives. The Compensation Committee intends to review our severance arrangements periodically to ensure that they remain necessary and appropriate.
Indemnification Agreements
We intend to enter into indemnification agreements with all of our executive officers. The indemnification agreements are governed exclusively by and construed according to the substantive laws of the Canada, without regard to conflicts-of-laws principles that would require the application of any other law, and provide, among other things, for indemnification, to the fullest extent permitted by law and our by-laws, against any and all expenses (including attorneys’ fees) and liabilities, judgments, fines and amounts paid in settlement that are paid or incurred by the executive or on his or her behalf in connection with such action, suit or proceeding. We will be obligated to pay these amounts only if the executive acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of our company and, in the case of a criminal or administrative proceeding that is enforced by a monetary penalty, he or she had reasonable grounds for believing that his or her conduct was lawful. The indemnification agreements provide that the executive will not be indemnified and expenses advanced with respect to an action, suit or proceeding initiated by the executive unless (i) so authorized or consented to by our Board of Directors or the company has joined in such action, suit or proceeding or (ii) the action, suit or proceeding is one to enforce the executive’s rights under the indemnification agreement. Our indemnification and expense advance obligations are subject to the condition that an appropriate person or body not party to the particular action, suit or proceeding shall not have determined that the executive is not permitted to be indemnified under applicable law. The indemnification agreements also set forth procedures that apply in the event an executive requests indemnification or an expense advance.
Summary Compensation Table
The table below provides summary information concerning all compensation awarded to, earned by or paid to our named executive officers during our 2017 and 2016 fiscal years. We did not have any officers during the year ended December 31, 2017, other than Rick Pauls and Todd Verdoorn, Ph.D.
|
Name and Principal Position |
|
Salary |
Bonus |
Option Awards(3) |
All Other Compensation(4) |
Total |
||||||||||||||||
|
Rick Pauls(1) |
2017 |
$ | 280,000 | $ | 36,667 | $ | 167,738 | $ | 17,550 | $ | 501,956 | |||||||||||
| President and Chief Executive Officer | 2016 | 276,250 | — | 100,196 | 11,400 | 387,846 | ||||||||||||||||
|
Todd Verdoorn, Ph.D.(2) |
2017 |
200,000 | 40,000 | 98,670 | 7,200 | 345,870 | ||||||||||||||||
| Chief Scientific Officer | 2016 | 164,792 | — | 58,939 | 4,250 | 227,981 | ||||||||||||||||
|
(1) |
Mr. Pauls is also a director of the company and did not receive any compensation related to his role as a director. |
|
(2) |
Dr. Verdoorn became a consultant to the company and was appointed as our Vice President of Neuroscience on January 20, 2016 and became an employee of the company and was promoted to Chief Scientific Officer on May 9, 2016. The portion of his 2016 salary for the period during which he served as a consultant was paid in the form of consulting fees. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
(3) |
Amounts reflect the full grant-date fair value of stock options granted during the applicable year computed in accordance with Accounting Standards Codification (ASC) Topic 718, rather than the amounts paid to or realized by the named individual. The grant date fair value is determined based on our Black-Scholes option pricing model. The table below sets forth the specific assumptions used in the valuation of each such option award: |
|
Grant Date |
Grant Date Fair Value Per Share ($) |
Risk Free Interest Rate |
Expected Life |
Expected Volatility |
Expected Dividend Yield |
|||||||||||||||
|
06/19/2017 |
$ | 0.248 | 0.98% | 4.4 years | 119.0% | — | ||||||||||||||
|
11/28/2016 |
0.158 | 1.01% |
|
5.5 years | 112.5% | — | ||||||||||||||
There can be no assurance that unvested awards will vest (and, absent vesting and exercise, no value will be realized by the executive for the award).
|
(4) |
The amounts shown in the “All Other Compensation” column for fiscal 2017 include the following with respect to each named executive officer: |
|
Name |
401(k) Match |
Health Savings Account Contribution |
Total |
|||||||||
|
Rick Pauls |
$ | 10,800 | $ | 6,750 | $ | 17,550 | ||||||
|
Todd Verdoorn, Ph.D. |
7,200 | — | 7,200 | |||||||||
Outstanding Equity Awards at Fiscal Year-End
The following table presents for each named executive officer information regarding outstanding equity awards held as of December 31, 2017.
|
Option Awards |
Stock Awards |
||||||||||||||||||||
|
Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable(1) |
Option Exercise Price CAD($) |
Option Expiration Date(2) |
Number of Shares or Units of Stock That Have Not Vested(3) |
Market Value of Shares or Units of Stock That Have Not Vested(4) ($) |
|||||||||||||||
|
Rick Pauls |
|||||||||||||||||||||
|
Stock Options |
200,000 | — | 1.15 |
10/06/2021 |
|||||||||||||||||
| 200,000 | — | 1.70 |
02/15/2022 |
||||||||||||||||||
| 200,000 | — | 1.07 |
06/25/2023 |
||||||||||||||||||
| 900,000 | 450,000 | 0.15 |
12/01/2025 |
||||||||||||||||||
| 283,333 | 566,667 | 0.26 |
11/28/2026 |
||||||||||||||||||
| 141,667 | 708,333 | 0.32 |
06/19/2027 |
||||||||||||||||||
|
DSUs |
34,985 | $ | 8,069 | ||||||||||||||||||
|
Todd Verdoorn, Ph.D. |
|||||||||||||||||||||
|
Stock Options |
96,000 | 48,0000 | 0.15 |
12/01/2025 |
— | — | |||||||||||||||
| 166,667 | 333,333 | 0.26 |
11/28/2026 |
||||||||||||||||||
| 83,333 | 416,667 | 0.32 |
06/19/2027 |
||||||||||||||||||
|
(1) |
All stock options vest in 12 equal quarterly installments over three years. |
|
(2) |
All stock options have a 10-year term, but may terminate earlier if the recipient’s employment or service relationship with our company terminates. |
|
(3) |
All DSU awards vest when the recipient’s employment or service relationship with our company terminates. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
(4) |
The market value of DSU awards that have not vested as of December 31, 2017 is based on the closing sale price of our common shares as reported by the TSX Venture Exchange on the last trading day of our fiscal year, December 29, 2017 (CAD$ to US$ fixed rate $0.7953). |
Director Compensation
The table below provides summary information concerning the compensation of each individual who served as a director of our company during the fiscal year ended December 31, 2017, other than Rick Pauls, our President and Chief Executive Officer, who was not compensated separately for serving on the Board of Directors during fiscal 2017. His compensation during fiscal 2017 for serving as an executive officer of our company is set forth under “—Summary Compensation Table.”
|
Name |
Fees Earned or Paid in Cash ($) |
Option Awards(1) ($) |
Stock Awards ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
|
Michael Giuffre, M.D. |
15,906 | 19,723 | — | — | 35,629 | |||||||||||||||
|
James Parsons |
15,906 | 19,723 | — | — | 35,629 | |||||||||||||||
|
Richard Pilnik |
31,812 | 19,723 | — | — | 51,535 | |||||||||||||||
|
Zhenyu Xiao |
15,906 | 19,723 | — | — | 35,629 | |||||||||||||||
|
(1) |
On June 19, 2017, each non-employee director received a stock option to purchase a 100,000 common shares at an exercise price of CAD$0.32 per share granted under our Stock Option Plan. Such option expires on June 19, 2027 and vests in 12 equal quarterly installments over three years. The amounts reflected represent the grant date fair value for option awards granted to each non-employee director computed in accordance with FASB ASC Topic 718. |
We use a combination of retainer fees and long-term equity-based incentive compensation in the form of stock option grants to attract and retain qualified candidates to serve on the Board of Directors. For fiscal 2017, each of our non-employee directors received annual retainers and meeting fees. Each non-employee director received a $13,918 annual retainer and the Chair of our Audit Committee and Compensation Committee received an additional $1,988 annual retainer. The Chairman of the Board received an additional $15,906. The annual retainers were accrued and unpaid as of December 31, 2017. All of our directors are reimbursed for travel expenses for attending meetings and other miscellaneous out-of-pocket expenses incurred in performing their Board functions.
For the reasons noted above, long-term equity based incentive compensation is a significant component of how we compensate directors. Directors generally receive annual grants with a fair market value equivalent to their cash compensation. These grants vest in 12 equal quarterly installments over three years and expire on the tenth anniversary of the grant date.
We intend to enter into indemnification agreements with all of our directors, which will be nearly identical to the indemnification agreements with our executive officers as described under “—Executive Compensation Overview—Indemnification Agreements.”
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 7. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
Related Person Relationships and Transactions
Other than as set describe below or under Item 6 “Executive Compensation,” we have not identified any transactions since January 1, 2016 to which we have been a party, in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets at year end for the last two fiscal years, and in which any of our executive officers, directors or holders of more than 5% of our common shares, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
Participation in Private Placement
On March 29, 2018, we completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on March 19, 2020 and March 29, 2020 for tranche 1 and tranche 2, respectively.
Rick Pauls, our President and Chief Executive Officer and a member of our Board of Directors, Scott Kellen, our Chief Financial Officer, and Michael Giuffre, M.D., a member of our Board of Directors, each participated in the offering on the same terms and conditions as other investors, as set forth in the table below:
|
Name |
Purchase Price |
Number of Common Shares |
Number of Common Shares Underlying Warrants |
|||||||||
|
Rick Pauls |
$ | 20,090 | 82,000 | 41,000 | ||||||||
|
Scott Kellen |
10,000 | 40,800 | 224,490 | |||||||||
|
Michael Giuffre, M.D. |
110,000 | 448,980 | 20,400 | |||||||||
|
Total |
$ | 140,090 | 571,780 | 285,890 | ||||||||
Relationship with Hermeda Industrial Co., Limited
We and Hermeda Industrial Co., Limited (“Hermeda”) are parties to an investment agreement, which includes terms relating to the composition of our Board of Directors. Under director nomination provisions of this agreement, Hermeda has the right to designate a representative to be nominated to our Board of Directors for so long as Hermeda beneficially owns at least 10% of our outstanding common shares on a non-diluted basis, and we agreed to use our reasonable best efforts to cause the Hermeda designee to be elected. As of August 20, 2018, Hermeda beneficially owned 12.8% of our outstanding common shares. Zhenyu Xiao, Ph.D., one of our directors, is the Managing Director of Hermeda and is the current designee of Hermeda under the investment agreement. In the event Hermeda has no representative on our Board of Directors and beneficially owns at least 10% of our outstanding common shares, on a non-diluted basis, and provides notice to us of its representative, we shall take such steps that are necessary for our Board of Directors to appoint the representative as a member of our Board of Directors.
To induce Hermeda to enter into the investment agreement, two members of our Board of Directors, Rick Pauls and Michael Giuffre, M.D., and certain of their related parties entered into voting agreements with DiaMedica pursuant to which these individuals agreed to vote their DiaMedica common shares in favor of the Hermeda designee to the Board of Directors at the then next annual general meeting of shareholders.
Director Independence
Following the effectiveness of this registration statement, our common shares will be listed on the Nasdaq Capital Market. Our Board of Directors has determined that all of our directors, other than Mr. Pauls, our President and Chief Executive Officer, are “independent directors” within the meaning of the rules of the Nasdaq Stock Market. Each member of our Audit Committee and Compensation Committee is an independent director within the meaning of the rules of the Nasdaq Stock Market and meets the standards for independence required by U.S. securities law requirements applicable to public companies, including Rule 10A-3 of the Exchange Act with respect to Audit Committee members and Rule 10C-1 under the Exchange Act, with respect to Compensation Committee members. In addition, each member of the Nominating and Corporate Governance Committee is also an independent director.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 8. |
LEGAL PROCEEDINGS. |
From time to time, we may be subject to various ongoing or threatened legal actions and proceedings, including those that arise in the ordinary course of business, which may include employment matters and breach of contract disputes. Such matters are subject to many uncertainties and to outcomes that are not predictable with assurance and that may not be known for extended periods of time. In the opinion of management, the outcome of such routine ongoing litigation is not expected to have a material adverse effect on our results of operations or financial condition.
|
ITEM 9. |
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS. |
Market Information
Our common shares currently trade in Canada on the TSX Venture Exchange under the trading symbol “DMA” and in the U.S. on the OTCQB Market under the trading symbol “DMCAF.” We have applied to list our common shares on Nasdaq under the trading symbol “DMAC.” The following table sets for the quarterly high and low market closing prices of our common shares on the TSX Venture Exchange and the OTCQB for the fiscal quarter indicated. We have converted the trading prices on the TSX Venture Exchange to U.S. dollars using the exchange rate on the date of the corresponding high or low sales price. In quarters in which the high or low sales price occurred on multiple dates the exchange rate for the latest occurrence is used for purposes of converting the U.S. dollar amount.
|
TSX Venture Exchange |
OTCQB |
|||||||||||||||||||||||
|
High (CAD$) |
High (US$) |
Low (CAD$) |
Low (US$) |
High (US$) |
Low (US$) |
|||||||||||||||||||
|
Fiscal 2018 |
||||||||||||||||||||||||
|
Third Quarter (through August 23, 2018) |
$ | 0.90 | $ | 0.69 | $ | 0.84 | $ | 0.64 | $ | 0.69 | $ | 0.63 | ||||||||||||
|
Second Quarter |
0.82 | 0.64 | 0.75 | 0.59 | 0.62 | 0.27 | ||||||||||||||||||
|
First Quarter |
.046 | 0.36 | 0.44 | 0.35 | 0.36 | 0.19 | ||||||||||||||||||
|
Fiscal 2017 |
||||||||||||||||||||||||
|
Fourth Quarter |
$ | 0.43 | $ | 0.33 | $ | 0.41 | $ | 0.32 | $ | 0.35 | $ | 0.23 | ||||||||||||
|
Third Quarter |
0.42 | 0.32 | 0.39 | 0.30 | 0.34 | 0.18 | ||||||||||||||||||
|
Second Quarter |
0.40 | 0.30 | 0.37 | 0.28 | 0.29 | 0.19 | ||||||||||||||||||
|
First Quarter |
0.30 | 0.23 | 0.27 | 0.20 | 0.21 | 0.113 | ||||||||||||||||||
|
Fiscal 2016 |
||||||||||||||||||||||||
|
Fourth Quarter |
$ | 0.24 | $ | 0.18 | $ | 0.24 | $ | 0.18 | $ | 0.20 | $ | 0.11 | ||||||||||||
|
Third Quarter |
0.35 | 0.26 | 0.32 | 0.24 | 0.26 | 0.18 | ||||||||||||||||||
|
Second Quarter |
0.33 | 0.25 | 0.30 | 0.23 | 0.25 | 0.11 | ||||||||||||||||||
|
First Quarter |
0.25 | 0.18 | 0.22 | 0.16 | 0.14 | 0.10 | ||||||||||||||||||
Nasdaq Stock Market Information
Upon the effectiveness of this registration statement, we intend to list our common shares on the Nasdaq Capital Market under the symbol “DMAC”.
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Trust Company.
Number of Record Holders
As of August 23, 2018, there were 65 record holders of our common shares. This does not include shares held in “street name” or beneficially owned.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Dividends
We do not currently expect to declare or pay dividends on our common shares for the foreseeable future. While there are no restrictions in our articles of continuance or elsewhere which would prevent us paying dividend, the Board of Directors intends to reinvest all available funds in the Company’s operations.
Securities Authorized for Issuance Upon the Exercise of Options and Warrants
As of August 15, 2018, there were outstanding options to purchase 12,549,689 of our common shares at a weighted average exercise price of CAD$0.39 per share warrants to purchase 16,628,865 of our common shares at a weighted average exercise price of $0.34 per share.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table summarizes outstanding options and other awards under our equity compensation plans as of August 15, 2018. Our equity compensation plans as of August 15, 2018 were the DiaMedica Therapeutics Inc. Stock Option Plan and the DiaMedica Therapeutics Inc. Deferred Share Unit Plan. All outstanding awards relate to our common shares.
|
(a) |
(b) |
(c) |
||||||||||
|
Plan category |
Number of Securities to be Issued Upon Exercise of Outstanding Options Warrants or Rights |
Weighted-Average Exercise Price of Outstanding Options, Warrants or Rights CAD($) |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
|
Equity compensation plans approved by security holders |
12,549,689(1) | 0.39(2) | 3,123,663(3) | |||||||||
|
Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
Total |
12,549,689(1) | 0.39(2) | 3,123,663(3) | |||||||||
|
(1) |
Amount includes common shares issuable upon exercise of stock options granted under the DiaMedica Therapeutics Inc. Stock Option Plan and common shares issuable upon the vesting of deferred share units granted under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan. |
|
(2) |
Excludes common shares under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan. |
|
(3) |
Amount includes 2,699,987 common shares remaining available for future issuance under the DiaMedica Therapeutics Inc. Stock Option Plan and 423,676 common shares remaining available for future issuance under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan. |
Equity Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register all common shares subject to outstanding stock options and shares of common stock issued or issuable under our stock plans. We expect to file the registration statement covering shares offered pursuant to our stock plans on or shortly after the effective date of this registration statement, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144.
Common Shares Not Registered Under the Securities Act
Our common shares, including our common shares underlying outstanding options and warrants, have not been registered under the Securities Act. Common shares which are not “restricted securities” under the Securities Act and are held by a shareholder that is not an affiliate of DiaMedica at the time of sale and has not been an affiliate of DiaMedica at any time during the three months preceding a sale, may be freely resold.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Rule 144
Common shares which are “restricted securities” and are held by a shareholder that is not an affiliate of DiaMedica at the time of sale and has not been an affiliate of DiaMedica at any time during the three months preceding a sale, and who has beneficially owned the shares for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our common shares for at least one year, such person can resell such shares under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Common shares held by a shareholder that is an affiliate of DiaMedica or has been an affiliate of DiaMedica at any time during the three months prior to the date of sale may only be sold under Rule 144 of the Securities Act beginning 90 days after the effectiveness of this registration statement, and subject to all other requirements of Rule 144. In general, under Rule 144, an affiliate would be entitled to sell in a “broker’s transaction” or certain “riskless principal transactions” or to market makers, a number of common shares within any three-month period that does not exceed the greater of: (i) 1% of the number of our common shares then outstanding; or (ii) the average weekly trading volume of our common shares on Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC and Nasdaq concurrently with either the placing of a sale order with the broker or the execution directly with a market maker. Persons who may be deemed to be our affiliates generally include individuals or entities that control, or are controlled by, or are under common control with, us and may include our directors and officers, as well as our significant shareholders.
Rule 701
Under Rule 701, common shares acquired upon the exercise of currently outstanding options or pursuant to other rights granted under our compensatory plans may be resold by:
|
● |
persons other than affiliates, beginning 90 days after the effective date of this registration statement, subject only to the manner-of-sale provisions of Rule 144; and |
|
● |
our affiliates, beginning 90 days after the effective date of this registration statement, subject to the manner-of-sale and volume limitations, current public information and filing requirements of Rule 144, in each case, without compliance with the six-month holding period requirement of Rule 144. |
Registration Rights
We have not agreed to register for sale under the Securities Act any common shares, including common shares issuable upon the exercise of any options or warrants, that we issued without registration under the Securities Act.
Canadian Resale Restrictions
As long as we continue to be considered a “reporting issuer” under applicable Canadian securities law, the sale of any of our common shares which constitutes a “control distribution” under applicable Canadian securities laws (generally a sale by a person or a group of persons holding 20% or more of our outstanding voting securities) must be qualified by a prospectus filed with Canadian securities regulatory authorities or, in the alternative, made in reliance on an applicable prospectus exemption. The sale of common shares pursuant to the use of a prospectus exemption will generally result in the sold common shares being subject to a four month hold period.
Other Canadian Laws Affecting U.S. Shareholders
There are no governmental laws, decrees or regulations in Canada relating to restrictions on the export or import of capital, or affecting the remittance of interest, dividends or other payments by us to non-residents of Canada. Dividends paid by the Company to residents of the United States of America within the meaning of the Canada-United States Tax Convention (1980) (the “Treaty”) are subject to a 15% withholding tax on the gross amount of the dividends (or a 5% withholding tax if the beneficial shareholder is a company which owns at least 10% of the outstanding voting common shares of the Company) pursuant to Article X of the Treaty. Dividends paid by the Company to other non-residents of Canada are subject to a 25% withholding tax on the amount of the dividends, unless reduced by an applicable tax treaty.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
There are no limitations specific to the rights of non-residents of Canada to hold or vote our common shares under the laws of Canada, or in our articles of continuance or by-laws, other than those imposed by the Investment Canada Act (Canada) as discussed below.
Non-Canadian investors who acquire a controlling interest in us may be subject to the Investment Canada Act (Canada), which governs the basis on which non-Canadians may invest in Canadian businesses. Under the Investment Canada Act (Canada), the acquisition of a majority of the voting interests of an entity (or of a majority of the undivided ownership interests in the voting common shares of an entity that is a corporation) is deemed to be an acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of the voting common shares. The acquisition of less than one-third of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is deemed not to be acquisition of control of that corporation.
|
ITEM 10. |
RECENT SALES OF UNREGISTERED SECURITIES. |
During the period beginning on January 1, 2018 through August 20, 2018, we granted to certain of our employees, directors and consultants 3,936,000 stock options and no DSUs. During the year ended December 31, 2017, we granted to certain of our employees, directors and consultants 2,552,689 stock options and no DSUs. During the year ended December 31, 2016, we granted to certain of our employees, directors and consultants 2,775,000 stock options and 375,000 DSUs. During the year ended December 31, 2015, we granted to certain of our employees, directors and consultants 4,404,000 stock options and no DSUs. These securities were issued under our equity incentive plans without registration under the Securities Act in reliance on the exemptions afforded by Section 4(a)(2) of the Securities Act and Rule 701 promulgated thereunder.
Set forth below is information regarding additional securities issued by us within the past three years that were not registered under the Securities Act. The offers, sales and issuances of the securities described below were deemed to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) (or Regulation D promulgated thereunder), in that the issuance of securities to the accredited investors did not involve a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor under Rule 501 of Regulation D. No underwriters were involved in these transactions.
|
1. |
On March 29, 2018, we completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on March 19, 2020 and March 29, 2020 for tranche 1 and tranche 2, respectively. The warrants are subject to early expiration under certain conditions. |
In connection with the offering, we paid an aggregate cash fee of approximately $384,000 to brokers and finders and issued an aggregate of approximately 1.6 million compensation options. Each compensation option entitles the holder to purchase one common share at $0.245, the offering price, for a period of two years from the closing of the offering, subject to acceleration on the same terms as the warrants issued to the investors.
|
2. |
On December 18, 2017, we completed a non-brokered private placement of 3,624,408 units at a price of $0.26 per unit for aggregate gross proceeds of approximately $944,000. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiration on December 19, 2019, subject to early expiration under certain conditions. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
3. |
On April 17, 2017, we completed a non-brokered private placement of 10,526,315 units at a price of $0.19 per unit for aggregate proceeds of approximately $2,000,000. Each unit consists of one common share and one-half common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.23 at any time prior to expiration on April 17, 2019. The warrant expiration date can be accelerated at our option in the event that the volume-weighted average trading price of our common shares exceeds $0.30 per common share for any 10 consecutive trading days. |
|
4. |
During the year ended December 31, 2017, 50,000 common shares were issued on the exercise of warrants for gross proceeds of $9,913 and 60,000 common shares were issued on the exercise of options for gross proceeds of $6,749. |
|
5. |
On September 8, 2016, we completed the second tranche of a non-brokered private placement of 15,000,000 common shares at a price of $0.20 per share for aggregate gross proceeds of $3,000,000. |
|
6. |
On August 22, 2016, we completed the first tranche of a non-brokered private placement of 5,000,000 common shares at a price of $0.20 per share for aggregate gross proceeds of $1,000,000. |
|
7. |
On April 22, 2016, we issued 50,000 common shares for settlement of a debt to a vendor at an issue price of CAD$0.20 per common share. |
|
8. |
On February 25, 2016, we completed the second tranche of a non-brokered private placement of 875,000 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $101,710. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitled the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiration of two years from the closing date. |
|
9. |
On February 18, 2016, we completed the first tranche of a non-brokered private placement of 3,812,500 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $445,544. Each unit consisted of one common share and one-half of one common share purchase warrant. Each whole warrant entitled the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiration of two years from the closing date. |
|
10. |
During the year ended December 31, 2016, 25,880 common shares were issued on the redemption of deferred share units and 3,482,150 common shares were issued on the exercise of warrants for gross proceeds of $617,212. |
|
11. |
On November 25, 2015, we announced the completion of a non-brokered private placement of 4,500,000 units at an issue price of $0.075 per unit for aggregate gross proceeds of $337,686. Each unit was comprised of one common share and one common share purchase warrant with each warrant entitling the holder thereof to acquire an additional common share at an exercise price of CAD$0.20 per share at any time prior to expiry on November 25, 2016. |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
|
ITEM 11. |
DESCRIPTION OF REGISTRANT’S SECURITIES TO BE REGISTERED. |
The following description of our equity securities does not purport to be complete and is subject, and qualified in its entirety by reference to, our articles of continuance and by-laws, each as amended, and applicable corporate and securities laws.
Authorized Equity Securities
We have an authorized share capital consisting of an unlimited number of voting common shares. As of August 20, 2018, there were 156,733,515 voting common shares issued and outstanding.
Certain Rights of the Common Shares
Dividends
Holders of our voting common shares are entitled to share pro rata in such dividends as may be declared by our Board of Directors. Pursuant to the provisions of the Canada Business Corporations Act, we may not declare or pay a dividend if there are reasonable grounds for believing that (1) we are, or would after the payment be, unable to pay our liabilities as they become due or (2) the realizable value of our assets would thereby be less than the aggregate of our liabilities and stated capital of all classes. We may pay a dividend by issuing fully paid shares, or in money or property.
Liquidation, Dissolution or Winding-Up
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company or any other distribution of our assets among our shareholders for the purpose of winding-up our affairs, holders of voting common shares are entitled to share pro rata in our assets available for distribution after we pay our creditors.
Voting Rights and Shareholders’ Meetings
Holders of our voting common shares are entitled to receive notice of and to attend and vote at all meetings of our shareholders. Each holder of our voting common shares is entitled to one vote, either in person or by proxy, on all matters submitted to shareholders.
Our Board of Directors must call an annual meeting of shareholders to be held not later than 15 months after the last preceding annual meeting of shareholders but no later than six months after the end of our preceding financial year end and may, at any time, call a special meeting of shareholders. For purposes of determining the shareholders who are entitled to receive notice of or to vote at a meeting of shareholders, the Board of Directors may, in accordance with the CBCA and National Instrument 54-101—Communications with Beneficial Owners of Securities of a Reporting Issuer of the Canadian Securities Administrators, fix in advance a date as the record date for that determination of shareholders, but that record date may not be more than 60 days or less than 30 days before the date on which the meeting is to be held.
The CBCA provides that notice of the time and place of a meeting of shareholders must be sent to each shareholder entitled to vote at the meeting, each director and to our auditors, not more than 60 days and not less than 21 days prior to the meeting. Under our by-laws, the presence at a shareholder meeting, in person or represented by proxy, of at least one shareholder holding not less than 10% of the outstanding voting common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting. A shareholder may participate in a meeting by means of telephone or other communication facilities that permit all persons participating in the meeting to communicate adequately with each other during the meeting
In the case of joint shareholders, one of the holders present at a meeting may, in the absence of the other holder(s) of the shares, vote the shares. If two or more joint shareholders are present in person or by proxy, then they are to vote as one on the shares held jointly by them.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
No Preemption Rights; Limited Restrictions on Directors’ Authority to Issue Common Shares
Existing holders of our voting common shares have no rights of preemption or first refusal under our articles of continuance, by-laws or the CBCA with respect to future issuances of our voting common shares. The voting common shares do not have conversion rights, are not subject to redemption and do not have the benefit of any sinking fund provisions. Subject to the rules and policies of The Nasdaq Stock Market and the TSX Venture Exchange and applicable corporate and securities laws, our Board of Directors has the authority to issue additional voting common shares.
Amendments to our Articles of Continuance and By-laws
Our articles of continuance, our by-laws and the CBCA govern the rights of holders of our shares.
Our shareholders can authorize the alteration of our articles of continuance to create additional classes of shares or to vary the rights or restrictions attached to any class of our shares by passing a special resolution approved by the holders of at least two-thirds of each class of affected shares represented in person or by proxy at a duly convened meeting of shareholders. Such a special resolution will not be effective until articles of amendment are filed with the Director appointed pursuant to the CBCA.
Our Board of Directors may, by resolution, make, amend or repeal any by-laws that regulate our business or affairs; provided that the Board of Directors shall submit a by-law, or an amendment or a repeal of a by-law, to the shareholders at the next meeting of the shareholders, and the shareholders may, by ordinary resolution, confirm, reject or amend the by-law, amendment or repeal. A by-law, or an amendment or a repeal of a by-law, is effective from the date of the resolution of the Board of Directors until it is confirmed, confirmed as amended or rejected by the shareholders.
Fundamental Changes
Pursuant to the CBCA, we may not effect any of the following fundamental changes without the consent of the holders of at least two-thirds of each class of our outstanding shares represented in person or by proxy and voting separately as a class at a duly convened meeting of our shareholders:
|
● |
any proposed amalgamation involving our company in respect of which the CBCA requires that the approval of our shareholders be obtained; |
|
● |
any proposed plan of arrangement pursuant to the CBCA involving our company in respect of which the CBCA or any order issued by an applicable court requires that the approval of our shareholders be obtained; |
|
● |
any proposed sale, lease or exchange of all or substantially all our assets or property; and |
|
● |
any dissolution, liquidation or winding-up of our company. |
Election and Removal of Directors
At each annual meeting of shareholders, our shareholders are required to elect directors to hold office for a term expiring not later than the close of the next annual meeting of shareholders. In accordance with our by-laws, any director who receives more “withhold” than “for” shareholder votes will be deemed to have tendered his or her resignation as a director. Our Board of Directors may fill vacancies among the Board.
Since shareholders do not have cumulative voting rights, holders of more than 50% of our outstanding common shares can elect all of our directors if they choose to do so. In such event, holders of the remaining shares will be unable to elect any director.
Under the CBCA, at least one quarter of our directors must be resident Canadians.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Anti-takeover Laws
In Canada, takeover bids are governed by provincial corporate and securities laws and the rules of applicable stock exchanges. The following description of the rules relating to acquisitions of securities and takeover bids to which Canadian corporate and securities laws apply does not purport to be complete and is subject, and qualified in its entirety by reference, to applicable corporate and securities laws, which may vary from province to province.
A party (the “acquiror”) who acquires beneficial ownership of, or control or direction over, more than 10% of the voting or equity securities of any class of a reporting issuer will generally be required to file with applicable provincial regulatory authorities both a news release and a report containing the information prescribed by applicable securities laws. Subject to the below, the acquiror (including any party acting jointly or in concert with the acquiror) will be prohibited from purchasing any additional securities of the class of the target company previously acquired for a period commencing on the occurrence of an event triggering the aforementioned filing requirement and ending on the expiry of one business day following the filing of the report. This filing process and the associated restriction on further purchases also apply in respect of subsequent acquisitions of 2% or more of the securities of the same class. The restriction on further purchases does not apply to an acquiror that beneficially owns, or controls or directs, 20% or more of the outstanding securities of that class.
In addition to the foregoing, certain other Canadian legislation may limit a Canadian or non-Canadian entity’s ability to acquire control over or a significant interest in us, including the Competition Act (Canada) and the Investment Canada Act (Canada). Issuers may also approve and adopt shareholder rights plans or other defensive tactics designed to be triggered upon the commencement of an unsolicited bid and make the company a less desirable takeover target.
Shareholder Rights Plan
We adopted a shareholder rights plan agreement (the “Rights Plan”). The Rights Plan is designed to provide adequate time for the Board of Directors and the shareholders to assess an unsolicited takeover bid for DiaMedica, to provide the Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The Rights Plan was renewed at the Company’s annual meeting of shareholders in December 2017 and is set to expire at the close of the Company’s annual meeting of shareholders in 2020.
The rights issued under the Rights Plan will initially attach to and trade with the common shares and no separate certificates will be issued unless an event triggering these rights occurs. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of the outstanding common shares without complying with the “Permitted Bid” provisions of the Rights Plan or without approval of the Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time.
Under the Rights Plan, a Permitted Bid is a bid made to all holders of the common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
The issuance of common shares upon the exercise of the rights is subject to receipt of certain regulatory approvals.
Listing; Exchange, Transfer Agent and Registrar
Our common shares currently trade in Canada on the TSX Venture Exchange under the trading symbol “DMA” and in the United States on the OTCQB Market under the trading symbol “DMCAF.” We have applied to list our common shares on Nasdaq under the trading symbol “DMAC.”
The transfer agent and registrar for our common shares is Computershare Trust Company.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Other Canadian Laws Affecting U.S. Shareholders
There are no governmental laws, decrees or regulations in Canada relating to restrictions on the export or import of capital, or affecting the remittance of interest, dividends or other payments by us to non-residents of Canada.
Dividends paid by the Company to residents of the United States of America within the meaning of the Canada-United States Tax Convention (1980) (the “Treaty”) are subject to a 15% withholding tax on the gross amount of the dividends (or a 5% withholding tax if the beneficial shareholder is a company which owns at least 10% of the outstanding voting common shares of the Company) pursuant to Article X of the Treaty. Dividends paid by the Company to other non-residents of Canada are subject to a 25% withholding tax on the amount of the dividends, unless reduced by an applicable tax treaty.
There are no limitations specific to the rights of non-residents of Canada to hold or vote our common shares under the laws of Canada, or in our articles of continuance or by-laws, other than those imposed by the Investment Canada Act (Canada) as discussed below.
Non-Canadian investors who acquire a controlling interest in us may be subject to the Investment Canada Act (Canada), which governs the basis on which non-Canadians may invest in Canadian businesses. Under the Investment Canada Act (Canada), the acquisition of a majority of the voting interests of an entity (or of a majority of the undivided ownership interests in the voting common shares of an entity that is a corporation) is deemed to be an acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of the voting common shares. The acquisition of less than one-third of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is deemed not to be acquisition of control of that corporation.
|
ITEM 12. |
INDEMNIFICATION OF DIRECTORS AND OFFICERS. |
We are a corporation organized under the Canada Business Corporations Act. Under Section 124 of the CBCA, a corporation may indemnify a present or former director or officer of the corporation or another individual who acts or acted at the corporation’s request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the corporation or other entity. A corporation may not indemnify an individual unless the individual (i) acted honestly and in good faith with a view to the best interests of the corporation, or, as the case may be, to the best interests of the other entity for which the individual acted as a director or officer or in a similar capacity at the corporation's request, and (ii) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the individual had reasonable grounds for believing that the conduct was lawful. Each of the aforementioned individuals are entitled to the indemnification provided above from a corporation as a matter of right if they were not judged by the court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done and if the individual fulfills conditions (i) and (ii) above. A corporation may advance moneys to a director, officer or other individual for the costs, charges and expenses of a proceeding; however, the individual shall repay the moneys if the individual does not fulfill the conditions set out in (i) and (ii) above. The indemnification or the advance of any moneys may be made in connection with a derivative action only with court approval and only if the conditions in (i) and (ii) above are met.
Under the CBCA, a corporation may purchase and maintain insurance for the benefit of any of the aforementioned individuals against any liability incurred by the individual in their capacity as a director or officer of the corporation, or in their capacity as a director or officer, or similar capacity, of another entity, if the individual acted in such capacity at the corporation’s request. We have maintained, and expect to continue to maintain, such an insurance policy covering our directors and officers with respect to certain liabilities.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
We intend to enter into indemnification agreements with all of our directors and officers. The indemnification agreements are governed exclusively by and construed according to the substantive laws of the Canada, without regard to conflicts-of-laws principles that would require the application of any other law and provide, among other things, for indemnification to the fullest extent permitted by law and our by-laws against any and all expenses (including attorneys’ fees) and liabilities, judgments, fines and amounts paid in settlement that are paid or incurred by the executive or on his or her behalf in connection with such action, suit or proceeding. We will be obligated to pay these amounts only if the executive acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of our company. The indemnification agreements provide that the executive will not be indemnified and expenses advanced with respect to an action, suit or proceeding initiated by the executive unless (i) so authorized or consented to by our Board of Directors or the company has joined in such action, suit or proceeding or (ii) the action, suit or proceeding is one to enforce the executive’s rights under the indemnification agreement. Our indemnification and expense advance obligations are subject to the condition that an appropriate person or body not party to the particular action, suit or proceeding shall not have determined that the executive is not permitted to be indemnified under applicable law. The indemnification agreements also set forth procedures that apply in the event an executive requests indemnification or an expense advance.
|
ITEM 13. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
See the financial statements and notes beginning on page F-1 of this registration statement.
|
ITEM 14. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
Not applicable.
|
ITEM 15. |
FINANCIAL STATEMENTS AND EXHIBITS.1 |
|
(a) |
Financial Statements |
See the index to consolidated financial statements set forth on page F-1.
|
(b) |
Exhibits |
The following documents are filed as exhibits hereto.
|
Exhibit No. |
Exhibit |
Method of Filing |
||
|
3.1 |
Certificate of Continuance of DiaMedica Therapeutics Inc. dated April 11, 2016 |
Filed herewith |
||
|
3.2 |
Certificate of Amendment of DiaMedica Therapeutics Inc. dated December 28, 2016 |
Filed herewith |
||
|
3.3 |
Certificate of Amendment of DiaMedica Therapeutics Inc. to reflect reverse stock split |
* |
||
|
3.4 |
By-Law No. 1A of DiaMedica Therapeutics Inc. as amended and restated on July 24, 2014 |
Filed herewith |
||
|
4.1 |
Investment Agreement between Hermeda Industrial Co., Ltd. and DiaMedica Inc. dated July 16, 2017 |
Filed herewith |
||
|
4.2 |
Filed herewith |
|||
|
4.3 |
Voting Agreement between Rick Pauls and DiaMedica Inc. dated July 2016 |
Filed herewith |
||
|
4.4 |
Voting Agreement between Werner Pauls and DiaMedica Inc. dated July 2016 |
Filed herewith |
||
|
4.5 |
Voting Agreement between Chris Pauls and DiaMedica Inc. dated July 2016 |
Filed herewith |
1 Fox to work with the company to compile required exhibits.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
* To be filed by amendment.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
SIGNATURES
In accordance with Section 12 of the Securities Exchange Act of 1934, the registrant caused this registration statement on Form 10 to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Dated: , 2018 |
DIAMEDICA THERAPEUTICS INC.
By: ____________________________________________ |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Audited Consolidated Financial Statements
|
Page |
|
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
Consolidated Balance Sheets as of December 31, 2017 and 2016 |
F-3 |
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2017 and 2016 |
F-4 |
|
Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2017 and 2016 |
F-5 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2017 and 2016 |
F-6 |
|
Notes to Consolidated Financial Statements |
F-7 |
Unaudited Condensed Consolidated Financial Statements for the Three Months Ended March 31, 2018 and 2017
|
Page |
|
|
Condensed Consolidated Balance Sheets as of March 31, 2018 and December 31, 2017 |
F-21 |
|
Condensed Consolidated Statements of Operations and Comprehensive Loss |
F-22 |
|
Condensed Consolidated Statements of Stockholders’ Equity |
F-23 |
|
Condensed Consolidated Statements of Cash Flows |
F-24 |
|
Notes to Condensed Consolidated Financial Statements |
F-25 |
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
DiaMedica Therapeutics Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of DiaMedica Therapeutics Inc. and Subsidiaries (the “Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, stockholders’ deficit, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years then ended in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company's Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s evaluations of the events and conditions and management’s plans regarding those matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board of the United States of America (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Baker Tilly Virchow Krause, LLP
We have served as the Company’s auditors since 2016.
Minneapolis, MN
August 24, 2018
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash |
$ | 1,353 | $ | 1,736 | ||||
|
Amounts receivable |
80 | 53 | ||||||
|
Prepaid expenses |
61 | 67 | ||||||
|
Total current assets |
1,494 | 1,856 | ||||||
|
Deposit |
271 | — | ||||||
|
Property and equipment, net |
37 | 19 | ||||||
|
Total non-current assets |
308 | 19 | ||||||
|
Total assets |
$ | 1,802 | $ | 1,875 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable and accrued liabilities |
$ | 919 | $ | 671 | ||||
|
Warrant liability |
84 | 93 | ||||||
|
Total current liabilities |
1,003 | 764 | ||||||
|
Commitments and contingencies (Note 10) |
||||||||
|
Stockholders’ equity: |
||||||||
|
Common shares, no par value; unlimited authorized; 127,413,262 and 110,520,960 shares issued and outstanding, as of December 31, 2017 and 2016, respectively |
— | — | ||||||
|
Additional paid-in capital |
41,033 | 37,085 | ||||||
|
Accumulated deficit |
(40,234 | ) | (35,974 | ) | ||||
|
Total stockholders’ equity |
799 | 1,111 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 1,802 | $ | 1,875 | ||||
See accompanying notes to consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
Year Ended December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Operating expenses: |
||||||||
|
Research and development |
$ | 3,206 | $ | 1,728 | ||||
|
General and administrative |
1,313 | 598 | ||||||
|
Operating loss |
(4,519 | ) | (2,326 | ) | ||||
|
Other (income) expense: |
||||||||
|
Governmental assistance - research incentives |
(244 | ) | — | |||||
|
Other (income) expense |
(6 | ) | 82 | |||||
|
Change in fair value of warrant liability |
(9 | ) | (188 | ) | ||||
|
Total other income |
(259 | ) | (106 | ) | ||||
|
Loss before income tax expense |
(4,260 | ) | (2,220 | ) | ||||
|
Income tax expense |
— | — | ||||||
|
Net loss and comprehensive loss |
$ | (4,260 | ) | $ | (2,220 | ) | ||
|
Basic and diluted net loss per share |
$ | (0.04 | ) | $ | (0.02 | ) | ||
|
Weighted average shares outstanding – basic and diluted |
118,715,801 | 94,715,025 | ||||||
See accompanying notes to consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In thousands except share amounts)
|
Additional |
Total |
|||||||||||||||
|
Common |
Paid-In |
Accumulated |
Stockholders’ |
|||||||||||||
|
Shares |
Capital |
Deficit |
Deficit |
|||||||||||||
|
Balances at December 31, 2015 |
82,275,430 | $ | 32,576 | $ | (33,754 | ) | $ | (1,178 | ) | |||||||
|
Issuance of common shares and warrants, net of offering costs of $395 |
20,000,000 | 3,605 | — | 3,605 | ||||||||||||
|
Issuance of common shares and warrants, net of offering costs of $311 |
4,687,500 | 237 | — | 237 | ||||||||||||
|
Issuance of common shares in settlement of debt |
50,000 | 8 | — | 8 | ||||||||||||
|
Exercise of common share warrants |
3,482,150 | 442 | — | 442 | ||||||||||||
|
Issuance of common shares, deferred stock unit redemption |
25,880 | — | — | — | ||||||||||||
|
Share-based compensation expense |
— | 217 | — | 217 | ||||||||||||
|
Net loss |
— | — | (2,220 | ) | (2,220 | ) | ||||||||||
|
Balances at December 31, 2016 |
110,520,960 | $ | 37,085 | $ | (35,974 | ) | $ | 1,111 | ||||||||
|
Issuance of common shares and warrants, net of offering costs of $292 |
14,150,723 | 2,917 | — | 2,917 | ||||||||||||
|
Exercise of common share purchase warrants |
2,681,579 | 615 | — | 615 | ||||||||||||
|
Exercise of common share options |
60,000 | 7 | — | 7 | ||||||||||||
|
Share-based compensation expense |
— | 409 | — | 409 | ||||||||||||
|
Net loss |
— | — | (4,260 | ) | (4,260 | ) | ||||||||||
|
Balances at December 31, 2017 |
127,413,262 | $ | 41,033 | $ | (40,234 | ) | $ | 799 | ||||||||
See accompanying notes to consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
Year Ended December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (4,260 | ) | $ | (2,220 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Share-based compensation |
409 | 217 | ||||||
|
Change in fair value of warrant liability |
(9 | ) | (188 | ) | ||||
|
Depreciation |
4 | 2 | ||||||
|
Changes in operating assets and liabilities: |
||||||||
|
Amounts receivable |
(27 | ) | (44 | ) | ||||
|
Prepaid expenses |
6 | (33 | ) | |||||
|
Deposits |
(271 | ) | — | |||||
|
Accounts payable and accrued liabilities |
248 | (510 | ) | |||||
|
Deferred revenue |
— | (39 | ) | |||||
|
Other liabilities |
— | (172 | ) | |||||
|
Net cash used in operating activities |
(3,900 | ) | (2,987 | ) | ||||
|
Cash flows from investing activities: |
||||||||
|
Purchase of property and equipment |
(22 | ) | (7 | ) | ||||
|
Net cash used in financing activities |
(22 | ) | (7 | ) | ||||
|
Cash flows from financing activities: |
||||||||
|
Proceeds from issuance of common shares and warrants, net of offering costs |
2,917 | 517 | ||||||
|
Proceeds from issuance of common shares, net of offering costs |
— | 3,605 | ||||||
|
Proceeds from the exercise of common share purchase warrants |
615 | 442 | ||||||
|
Proceeds from the exercise of stock options |
7 | — | ||||||
|
Net cash provided by financing activities |
3,539 | 4,564 | ||||||
|
Net (decrease) increase in cash |
(383 | ) | 1,570 | |||||
|
Cash at beginning of year |
1,736 | 166 | ||||||
|
Cash at end of year |
$ | 1,353 | $ | 1,736 | ||||
|
Supplemental disclosure of non-cash transactions: |
||||||||
|
Common share purchase warrants issued as agent consideration |
$ | — | $ | 24 | ||||
|
Common shares issued in settlement of debt |
$ | — | $ | 8 | ||||
See accompanying notes to consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Notes to Consolidated Financial Statements
1. Business
DiaMedica Therapeutics Inc. and its wholly-owned subsidiaries, DiaMedica USA, Inc. and DiaMedica Australia Pty Ltd. (collectively “we,” “us,” “our” and the “Company”), exist for the primary purpose of advancing the clinical and commercial development of a proprietary recombinant KLK1 protein for the treatment of acute ischemic stroke and chronic kidney disease.
The Company is a listed company incorporated under the Canada Business Corporations Act and domiciled in British Columbia, Canada, whose shares are publicly traded on the TSX Venture Exchange in Canada under the symbol “DMA” and the OTCQB in the United States under the symbol “DMCAF.” The Company’s registered office is at 301 – 1665 Ellis Street, Kelowna, British Columbia V1Y 2B3. DiaMedica USA Inc. was incorporated under the laws of the State of Delaware on May 15, 2012. DiaMedica Australia Pty Ltd. was established on July 11, 2016 and incorporated under the laws of Australian Securities and Investments Commission.
2. Risks and Uncertainties
The Company operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (“FDA”) in the United States, the Therapeutic Goods Administration (“TGA”) in Australia, the European Medicines Agency (“EMA”) in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditures.
As of December 31, 2017, we have incurred losses of $40.2 million since our inception in 2000. For the year ended December 31, 2017, we incurred a net loss and negative cash flows from operating activities of $4.3 million and $3.9 million, respectively. We expect to incur substantial losses for the foreseeable future, which will continue to generate negative net cash flows from operating activities, as we continue to pursue research and development activities and the clinical development of our primary product candidate, DM199. As of December 31, 2017, we had cash of $1.4 million, working capital of $491,000 and stockholders’ equity of $799,000. The Company’s principal sources of cash have included the issuance of equity securities.
The accompanying Consolidated Financial Statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our initial product candidate, DM199, in the United States, Australia, the European Union or other markets and ultimately our ability to market and sell our initial product candidate. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See Note 3 titled “Liquidity, Management’s Plans and Going Concern.”
3. Liquidity, Management Plans and Going Concern
As of December 31, 2017 and March 31, 2018, the Company has an accumulated deficit of $40.2 million and $40.9 million, respectively, and the Company has not generated positive cash flow from operations since its inception.
Additional funding will be required to continue the Company’s research and development and other operating activities. In the next 12 months we will likely seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data is not positive or economic and market conditions deteriorate.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
During March 2018, the Company completed a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. In addition, during February 2018, 2,425,125 common shares were issued on the exercise of warrants for gross proceeds of approximately $484,000. See Note 14 titled “Subsequent Events” for further details.
If we are unable to obtain additional financing when needed, we would need to scale back our operations taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modify or delay the development of our DM199 product candidate, license to third parties the rights to commercialize our DM199 product candidate for acute ischemic stroke, chronic kidney disease or other applications that we would otherwise seek to pursue, or cease operations.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to demonstrate clinical progress for our DM199 product candidate in the United States or other markets, our ability to obtain required governmental approvals of our product candidate and ultimately our ability to license or market and sell our DM199 product candidate. If we are unable to obtain additional financing when needed, if our clinical trials are not successful, if we are unable to obtain required governmental approvals, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional equity securities would likely result in dilution to our current stockholders.
4. Summary of Significant Accounting Policies
Basis of presentation
We have prepared the accompanying Consolidated Financial Statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) which contemplates the realization of its assets and the settlement of its liabilities in the normal course of operations. Our fiscal year ends on December 31.
Principles of consolidation
The accompanying Consolidated Financial Statements include the assets, liabilities and expenses of DiaMedica Therapeutics Inc. and our wholly-owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Functional currency
The United States dollar is the functional currency that represents the economic effects of the underlying transactions, events and conditions and various other factors including the currency of historical and future expenditures and the currency in which funds from financing activities are mostly generated by the Company. A change in the functional currency occurs only when there is a material change in the underlying transactions, events and condition. A change in functional currency could result in material differences in the amounts recorded in the consolidated statement of loss and comprehensive loss for foreign exchange gains and losses. All amounts in the accompanying Consolidated Financial Statements are in U.S. dollars unless otherwise indicated.
Use of estimates
The preparation of Consolidated Financial Statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentration of credit risk
Financial instruments that potentially subject the company to significant concentrations of credit risk consist primarily of cash. Cash is deposited in demand accounts at commercial banks. At times, such deposits may be in excess of insured limits. The Company has not experienced any losses on its deposits of cash.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Fair value of financial instruments
Carrying amounts of certain of the Company’s financial instruments, including amounts receivable, prepaid expenses and other current assets, accounts payable and accrued liabilities approximate fair value due to their short maturities. Certain of the Company’s common share purchase warrants are required to be reported at fair value. The fair value of common share purchase warrants is disclosed in Note 9 titled “Warrant Liability.”
Fair value measurements
Fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
The hierarchy is broken down into three levels defined as follows:
|
● |
Level 1—Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
|
● |
Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active or model-derived valuations for which all significant inputs are observable, either directly or indirectly. |
|
● |
Level 3—Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable. |
Our cash and equivalents consist of bank deposits. As of December 31, 2017, the Company believes that the carrying amounts of its other financial instruments, including amounts receivable, accounts payable and accrued liabilities, approximate their fair value due to the short-term maturities of these instruments.
Common share warrant liability
The common share warrants that were issued in connection with the February 2016 private placements of common shares are classified as a liability in the consolidated balance sheets, as the common share warrants have an exercise price stated in Canadian dollars, which is different than the functional currency, and thus these warrants qualify as a derivative instruments. The fair value of these common share warrants is re-measured at each financial reporting period and immediately before exercise, with any changes in fair value being recognized as a component of other income (expense) in the consolidated statements of operations.
Long-lived assets
Property and equipment are stated at purchased cost less accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of three to ten years for office equipment and four years for computer equipment. Upon retirement or sale, the cost and related accumulated depreciation are removed from the consolidated balance sheets and the resulting gain or loss is reflected in the consolidated statements of operations. Repairs and maintenance are expensed as incurred.
Long-lived assets are evaluated for impairment when events or changes in circumstances indicate that the carrying amount of the asset or related group of assets may not be recoverable. If the expected future undiscounted cash flows are less than the carrying amount of the asset, an impairment loss is recognized at that time. Measurement of impairment may be based upon appraisal, market value of similar assets or discounted cash flows.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Research and development costs
Research and development costs include expenses incurred in the conduct of human clinical trials, for third-party service providers performing various testing and accumulating data related to non-clinical studies; sponsored research agreements; developing the manufacturing process necessary to produce sufficient amounts of the DM199 compound for use in our non-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our DM199 product candidate; and personnel costs, including salaries, benefits and share-based compensation.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual work performed at each clinical trial site and by each CRO.
Patent costs
Costs associated with prosecuting and maintaining patents are expensed as incurred given the uncertainty of patent approval and, if approved, resulting in probable future economic benefit to the Company. Patent-related costs, including legal expenses, included in research and development costs were $160,000 and $45,000 for the years ended December 31, 2017 and 2016, respectively.
Share-based compensation
The cost of employee and non-employee services received in exchange for awards of equity instruments is measured and recognized based on the estimated grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the vesting period, which is considered to be the requisite service period. We record forfeitures in the periods in which they occur.
The fair value of share-based awards is estimated at the date of grant using the Black-Scholes option pricing model. The determination of the fair value of share-based awards is affected by our stock price, as well as assumptions regarding a number of complex and subjective variables. Risk free interest rates are based upon Canadian Government bond rates appropriate for the expected term of each award. Expected volatility rates are based on the on historical volatility equal to the expected life of the option. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past.
Income taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the Consolidated Financial Statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted rates, for each of the jurisdictions in which the Company operates, expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that is more likely than not to be realized. The Company has provided a full valuation allowance against the gross deferred tax assets as of December 31, 2017 and 2016. See Note 13, “Income Taxes” for additional information. The Company’s policy is to classify interest and penalties related to income taxes as income tax expense in the Consolidated Statements of Operations and Comprehensive Loss.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Government assistance
Government assistance relating to research and development performed by DiaMedica Australia Pty Ltd. is recorded as a component of Other (Income) Expense. Government assistance is initially recognized when reasonable assurance exists that the Company will comply with the conditions attached to the incentive program and that the incentive payments will be received. In subsequent periods, the government assistance is recognized when the related expenditures are incurred.
Net loss per share
We compute net loss per share by dividing our net loss (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period, if any, are weighted for the portion of the period that they were outstanding. The computation of diluted earnings per share, or diluted EPS, is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Our diluted EPS is the same as basic EPS due to common equivalent shares being excluded from the calculation, as their effect is anti-dilutive.
The following table summarizes our calculation of net loss per common share for the periods (in thousands, except share and per share data):
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Net loss |
$ | (4,260 | ) | $ | (2,220 | ) | ||
|
Weighted average shares outstanding—basic and diluted |
118,715,801 | 94,715,025 | ||||||
|
Basic and diluted net loss per share |
$ | (0.04 | ) | $ | (0.02 | ) | ||
The following outstanding potential common shares were not included in the diluted net loss per share calculations as their effects were not dilutive:
|
Year Ended December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Employee and non-employee stock options |
9,600,689 | 8,557,000 | ||||||
|
Common shares issuable under common share purchase warrants |
4,324,254 | 2,562,050 | ||||||
|
Common shares issuable under deferred share unit plan |
423,676 | 423,676 | ||||||
| 14,348,619 | 11,542,726 | |||||||
Recently issued accounting pronouncement
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases. The guidance in ASU 2016-02 supersedes the lease recognition requirements in the Accounting Standards Codification Topic 840, Leases. ASU 2016-02 requires an entity to recognize assets and liabilities arising from a lease for both financing and operating leases, along with additional qualitative and quantitative disclosures. The new standard requires the immediate recognition of all excess tax benefits and deficiencies in the income statement and requires classification of excess tax benefits as an operating activity as opposed to a financing activity in the statements of cash flows. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, with early adoption permitted. Management is evaluating the standard's impact on the Consolidated Financial statements.
In June 2018, the FASB issued ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which simplifies several aspects of the accounting for nonemployee share-based payment transactions resulting from expanding the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. This ASU is effective for the Company for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. The Company is currently evaluating the impact of the new guidance on our Consolidated Financial Statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Recently adopted accounting pronouncements
In March 2016, the FASB issued ASU No. 2016-09, Improvements to Employee Share-Based Payment Accounting. The guidance in ASU 2016-09 is intended to simplify aspects of the accounting for employee share-based payments, including the accounting for income taxes, forfeitures, statutory withholding requirements, and classification on the statement of cash flows. The standard is effective for interim and annual periods beginning after December 15, 2016, with early adoption permitted. The adoption of ASU 2016-09 during the year ended December 31, 2016 did not have a material impact on the Consolidated Financial Statements and related disclosures.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features; and II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Non-controlling Interests with a Scope Exception. Part I of this update addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments (such as warrants and convertible instruments) with down round features that require fair value measurement of the entire instrument or conversion option. Part II of this update addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification. This pending content is the result of the indefinite deferral of accounting requirements about mandatorily redeemable financial instruments of certain nonpublic entities and certain mandatorily redeemable non-controlling interests. The amendments in Part II of this update do not have an accounting effect. This ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. The Company adopted ASU 2017-11 during the year ended December 31, 2017. Due to the adoption, the December 2017 warrants were not accounted for as derivative instruments. There was no activity in prior years which fall under this guidance. As such, early adoption has no effect on prior years.
5. Amounts Receivable
Amounts receivable consisted of the following (in thousands):
|
December 31, 2017 |
December 31, 2016 |
|||||||
|
Sales-based taxes receivable |
80 | 53 | ||||||
|
Total amounts receivable |
$ | 80 | $ | 53 | ||||
6. Deposit
Deposit consisted of the following (in thousands):
|
December 31, 2017 |
December 31, 2016 |
|||||||
|
Advances to vendor |
$ | 271 | $ | — | ||||
|
Total Deposit |
$ | 271 | $ | — | ||||
We have advanced funds to a vendor engaged to support the performance of the REMEDY Phase 2 clinical trial. The funds advanced will be held, interest free, by this vendor until the completion of the trial and applied to final trial invoices or refunded. This deposit is classified as non-current as the trial is not expected to be completed during 2018.
7. Property and Equipment
Property and equipment consisted of the following (in thousands):
|
December 31, 2017 |
December 31, 2016 |
|||||||
|
Furniture and equipment |
$ | 40 | $ | 22 | ||||
|
Computer equipment |
23 | 20 | ||||||
| 63 | 42 | |||||||
|
Less accumulated depreciation |
(26 | ) | (23 | ) | ||||
|
Property and equipment, net |
$ | 37 | $ | 19 | ||||
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
8. Accrued Liabilities
Accounts payable and accrued liabilities consisted of the following (in thousands):
|
December 31, 2017 |
December 31, 2016 |
|||||||
|
Trade and other payables |
$ | 513 | $ | 250 | ||||
|
Accrued compensation and related |
355 | 142 | ||||||
|
Accrued research and other professional fees |
45 | 255 | ||||||
|
Other accrued liabilities |
6 | 24 | ||||||
|
Total accrued liabilities |
$ | 919 | $ | 671 | ||||
9. Warrant Liability
In February 2016, the Company completed, in two tranches, a non-brokered private placement of 4,687,500 units with each unit consisting of one common share and one half of one common share purchase warrant. The Company issued 2,343,750 warrants. Each warrant entitles the holder to purchase one common share at a price of $0.25 Canadian dollars at any time prior to expiry on February 18 or 25, 2018 for Tranche 1 and Tranche 2, respectively.
As the warrant exercise price is stated in Canadian dollars and the Company’s functional currency is the U.S. dollar, the warrants are deemed to be a derivative, with their estimated fair value classified as a liability on the Company’s balance sheet. The initial estimated fair value of the warrants was recorded as a warrant liability with subsequent changes in the estimated fair value recognized in the Consolidated Statements of Operations and Comprehensive Loss. The Company allocated $257,000 of the net proceeds to the warrant liability and the balance of the proceeds to the common shares (Note 9). The initial fair value of the warrants was determined using a Black-Scholes pricing model with the following assumptions: expected volatilities of 191.8 – 225.0%, risk-free interest rates of 0.43 – 0.49%, and expected life of 2 years.
In connection with the offering, the Company issued an aggregate of 218,300 compensation warrants. Each compensation warrant entitles the holder to purchase one common share at $0.25 Canadian dollars for a period of 2 years from the date of issuance, subject to acceleration on the same terms as the common share purchase warrants. The Company estimated the value of these warrants at $24,000, which was included in the issuance costs. The initial fair value of the warrants was determined using a Black-Scholes pricing model with the following assumptions: expected volatilities of 191.8 – 225.0%, risk-free interest rates of 0.43 – 0.49%, and expected life of 2 years.
The fair value of the Company’s common share purchase warrant liability, for both investor warrants and compensation warrants, is calculated using a Black-Scholes valuation model and is classified as Level 3 in the fair value hierarchy. The fair values were estimated using the following valuation assumptions:
|
Unit Warrants December 31, |
Compensation Warrants December 31, |
||||||
|
2017 |
2016 |
2017 |
2016 |
||||
|
Common share fair value |
$0.26 – $0.42 |
$0.16 – $0.24 |
$0.26 – $0.42 |
$0.16 – $0.24 |
|||
|
Risk-free interest rate |
0.75% – 1.67% |
0.43% – 0.76% |
0.75% – 1.67% |
0.43% – 0.76% |
|||
|
Expected dividend yield |
0% |
0% |
0% |
0% |
|||
|
Expected life (years) |
0.13 – 0.89 |
1.1 – 2.0 |
0.13 – 0.89 |
1.1 – 2.0 |
|||
|
Expected stock price volatility |
20.8% – 105.3% |
89.6% – 191.8% |
20.8% – 105.3% |
89.6% – 191.8% |
|||
The following is a rollforward of the fair value of Level 3 warrants (in thousands):
|
Warrant Liability |
||||
|
Warrant issuance – February 2016 |
$ | 281 | ||
|
Change in fair value |
(188 | ) | ||
|
Ending balance December 31, 2016 |
93 | |||
|
Change in fair value |
(9 | ) | ||
|
Ending balance December 31, 2017 |
$ | 84 | ||
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
10. Commitments and Contingencies
Clinical trials and product development
In the normal course of business, the Company incurs obligations to make future payments as it executes its business plan. These contracts relate to preclinical, clinical and development activities, including the clinical research organization conducting our Phase II clinical trial for acute ischemic stroke. These commitments are subject to significant change and the ultimate amounts due may be materially different as these obligations are affected by, among other factors, the number and pace of patients enrolled, the number of clinical study sites, amount of time to complete study enrollments and the time required to finalize the analysis and reporting of study results. Clinical research agreements are generally cancelable upon 30 days notice, with the Company’s obligation then limited to costs incurred up to that date. Cancelation terms for product development contracts vary and are generally dependent upon timelines for sourcing research materials and reserving laboratory time. As of December 31, 2017, the Company estimates that its outstanding commitments including research and development contracts are approximately $2.2 million over the next 12 months and approximately $700,000 in the following 12 months.
On September 11, 2017, the Company announced the initiation of REMEDY, a 60-patient Phase II clinical trial evaluating DM199 in patients with acute ischemic stroke (“AIS”). The study drug (DM199 or placebo) will be administered as an intravenous (“IV”) infusion within 24 hours of stroke symptom onset, followed by subcutaneous (under the skin) injections once every 3 days for 21 days. The study is designed to measure safety and tolerability along with multiple tests designed to investigate DM199’s therapeutic potential including plasma-based biomarkers and standard functional stroke measures assessed at 90 days post-stroke (Modified Rankin Scale (“MRS”), National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI), and CRP, a measure of inflammation).
Additional clinical trials will be subsequently required if the results of the Phase II are positive. However, at this time, we are unable to reasonably estimate the total costs of future trials. Such costs are dependent upon and subject to change depending on the results of current and future clinical trials as well as developments in the regulatory requirements. Clinical trial costs are expensed as incurred.
Technology license
The Company has entered into a research, development, and license agreement whereby the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under this agreement with such payments dependent upon, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain. There were no amounts due or payable under this agreement during 2017 and 2016.
Indemnification of directors and officers
The Company, as permitted under laws of the Canada and in accordance with its by-laws, will indemnify and advance expenses to its directors and officers to the fullest extent permitted by law or, if applicable, pursuant to indemnification agreements. They further provide that we may choose to indemnify other employees or agents of our Company from time to time. The Company has secured insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to the Company. As of December 31, 2017, there was no pending litigation or proceeding involving any director or officer of the Company as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company, the Company has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
The Company believes the fair value of these indemnification agreements is minimal. Accordingly, the Company had not recorded any liabilities for these obligations as of December 31, 2017 or 2016.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Future minimum lease payments
The Company leases certain office space under a non-cancelable operating lease. On May 3, 2017, the Company amended the lease agreement to extend its lease term by 42 months, for an expiration date of August 31, 2022, and increase its leased space. Rent is expensed on a straight-line basis.
Future minimum lease payment under this operating lease are as follows (in thousands):
|
2018 |
$ | 62 | ||
|
2019 |
64 | |||
|
2020 |
66 | |||
|
2021 |
68 | |||
|
2022 |
45 | |||
| $ | 305 |
11. Stockholders’ Deficit
Authorized capital stock
The Company has authorized share capital of an unlimited number of common voting shares and the shares have no stated par value.
Common shareholders are entitled to receive dividends as declared by the Company, if any, and are entitled to one vote per share at the Company's annual general meeting and any extraordinary general meeting.
Shareholders rights plan
The Company adopted a shareholder rights plan agreement (the “Rights Plan”). The Rights Plan is designed to provide adequate time for the Board of Directors and the shareholders to assess an unsolicited takeover bid for DiaMedica, to provide the Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The Rights Plan was renewed at the Company’s annual meeting of shareholders in December 2017 and is set to expire at the close of the Company’s annual meeting of shareholders in 2020.
The rights issued under the Rights Plan will initially attach to and trade with the common shares and no separate certificates will be issued unless an event triggering these rights occurs. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20 percent (20%) or more of the outstanding common shares without complying with the “Permitted Bid” provisions of the Rights Plan or without approval of the Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50 percent (50%) discount to the market price at the time.
Under the Plan, a Permitted Bid is a bid made to all holders of the common shares and which is open for acceptance for not less than sixty (60) days. If at the end of sixty (60) days at least 50 percent (50%) of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further ten (10) days to allow other shareholders to tender.
The issuance of common shares upon the exercise of the rights is subject to receipt of certain regulatory approvals.
Private placements during 2017
On December 18, 2017, the Company completed a non-brokered private placement of 3,624,408 units at a price of $0.26 per unit for aggregate gross proceeds of approximately $944,000, or $934,000 net of issuance costs. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiry on December 19, 2019. Warrants are subject to early expiry, at the option of the Company, if on any date the volume-weighted average closing trading price of the common shares on any recognized Canadian stock exchange equals or exceeds $0.60 for a period of 21 consecutive trading days.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
On April 17, 2017, the Company completed a non-brokered private placement of 10,526,315 units at a price of $0.19 per unit for aggregate gross proceeds of approximately $2,000,000, or $1,983,000 net of issuance costs. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.23 at any time prior to expiry on April 17, 2019. Warrants are subject to early expiry, at the option of the Company, if on any date the volume-weighted average closing trading price of the common shares on any recognized Canadian stock exchange equals or exceeds $0.30 for a period of 10 consecutive trading days.
During the year ended December 31, 2017, 2,681,579 common shares were issued on the exercise of warrants for gross proceeds of $615,000 and 60,000 common shares were issued on the exercise of options for gross proceeds of $7,000.
Private placements during 2016
On August 22, 2016 and September 8, 2016, the Company completed a non-brokered private placement of 15,000,000 and 5,000,000 common shares, respectively, at a price of $0.20 per share for aggregate gross proceeds of $4,000,000, or $3,605,000 net of issuance costs.
On April 22, 2016, the Company issued 50,000 common shares for settlement of a debt to a vendor at an effective issue price of approximately $0.16 per common share.
On February 25, 2016, the Company completed the second tranche of a non-brokered private placement of 875,000 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $102,000. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiry of February 25, 2018. In connection with the financing, the Company issued 70,000 compensation warrants and paid a finder’s fee of 8% of the aggregate gross proceeds. Each compensation warrant entitles the holder to acquire one common share at an exercise price of CAD$0.25 prior to expiry on February 25, 2018.
The proceeds from the sale were allocated first to the warrants as a derivative liability and the remainder to the common shares. As a result, approximately $52,000 of the proceeds were allocated to the warrant derivative liability and the remaining proceeds of approximately $50,000, before offering costs, were allocated to the common shares.
On February 18, 2016, the Company completed the first tranche of a non-brokered private placement of 3,812,500 units at a price of $0.117 per unit for aggregate gross proceeds of approximately $446,000. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of CAD$0.25 at any time prior to expiry on February 18, 2018. In connection with the financing, the Company issued 148,300 compensation warrants and paid a net finder’s fee of 4% of the aggregate gross proceeds. Each compensation warrant entitles the holder to acquire one common share at an exercise price of CAD$0.25 prior to expiry on February 18, 2018.
The proceeds from the sale were allocated first to the warrants as a derivative liability and the remainder to the common shares. As a result, approximately $205,000 of the proceeds were allocated to the warrant derivative liability and the remaining proceeds of approximately $240,000, before offering costs, were allocated to the common shares.
During the year ended December 31, 2016, 25,880 common shares were issued on the redemption of deferred share units and 3,482,150 common shares were issued on the exercise of warrants for gross proceeds of $442,000, and 10,891,087 warrants expired unexercised.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Shares reserved
Shares of common stock reserved for future issuance are as follows:
|
December 31, 2017 |
||||
|
Stock options outstanding |
9,600,689 | |||
|
Deferred share units outstanding |
423,676 | |||
|
Shares available for grant under the DiaMedica Stock Option Plan |
4,324,254 | |||
|
Common shares issuable under common stock purchase warrants |
3,140,637 | |||
|
Total |
17,489,256 | |||
12. Share-based Compensation
Deferred share unit plan
The 2012 Deferred Share Unit Plan (the “2012 DSU Plan”) promotes greater alignment of long-term interests between non-executive directors and executive officers of the Company and its shareholders through the issuance of deferred share units (“DSUs”). Since the value of DSUs increases or decreases with the market price of the common shares, DSUs reflect a philosophy of aligning the interests of directors and executive officers by tying compensation to share price performance. For the years ended December 31, 2017 and 2016, there were zero and 375,000 shares issued, respectively, with an intrinsic value of zero and $53,000, respectively, for payment of directors’ fees. The Company has reserved for issuance up to 2,000,000 common shares under the 2012 DSU Plan and 423,676 DSUs were outstanding at December 31, 2017 and 2016.
Stock option plan
DiaMedica has adopted a Stock Option Plan (the “Option Plan”) where the Board of Directors may from time to time, in their sole discretion, and in accordance with the requirements of the Toronto (TSX) Venture Exchange, grant to directors, officers, management company employees, investor relations consultants and Consultants (as such terms are used in the Stock Option Plan) to DiaMedica, non-transferable options to purchase common shares. The shareholders approved the adoption of an Option Plan on September 22, 2011, and as amended and restated on October 23, 2015 and December 21, 2017, reserving for issuance up to 10% of the Company’s issued and outstanding common shares. Options granted vest at various rates and have terms of up to 10 years. As of December 31, 2017, options to purchase 9,600,689 common shares were outstanding. As the TSX Venture Exchange is the principle trading market for the Company’s shares, all options have been priced in Canadian dollars.
The aggregate number of common shares reserved as of December 31, 2017 was 12,741,000, which includes both the Option Plan and the 2012 DSU Plan.
We recognize share-based compensation based on the fair value of each award as estimated using the Black-Scholes option valuation model. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
A summary of option activity is as follows:
|
Shares Underlying Options |
Weighted Average Exercise Price Per Share (CAD$) |
Aggregate Intrinsic Value (CAD$) |
||||||||||
|
Balances at December 31, 2015 |
6,412,000 | $ | 0.49 | $ | 60,000 | |||||||
|
Shares Reserved |
— | — | ||||||||||
|
Granted |
2,775,000 | 0.24 | ||||||||||
|
Exercised |
— | — | ||||||||||
|
Expired / cancelled |
(480,000 | ) | 0.72 | |||||||||
|
Forfeited |
(150,000 | ) | 1.31 | |||||||||
|
Balances at December 31, 2016 |
8,557,000 | $ | 0.38 | $ | 187,120 | |||||||
|
Granted |
2,552,689 | 0.31 | ||||||||||
|
Exercised |
(60,000 | ) | 0.15 | |||||||||
|
Expired / cancelled |
(1,449,000 | ) | 0.66 | |||||||||
|
Forfeited |
— | — | ||||||||||
|
Balances at December 31, 2017 |
9,600,689 | $ | 0.32 | $ | 674,481 | |||||||
A summary of the status of our unvested shares during the year ended and as of December 31, 2017 is as follows:
|
Shares Under Option |
Weighted Average Grant-Date Fair Value |
|||||||
|
Unvested at December 31, 2016 |
298,400 | $ | 9.47 | |||||
|
Granted |
54,000 | 9.48 | ||||||
|
Vested |
(217,200 | ) | 8.79 | |||||
|
Forfeitures |
— | — | ||||||
|
Unvested at December 31, 2017 |
135,200 | $ | 9.31 | |||||
Information about stock options outstanding, vested and expected to vest as of December 31, 2017, is as follows:
|
Outstanding, Vested and Expected to Vest |
Options Vested and Exercisable |
|||||||||||||||||||
|
Weighted Average |
Weighted |
Weighted Average |
||||||||||||||||||
|
Per Share |
Remaining |
Average |
Remaining |
|||||||||||||||||
|
Exercise Price |
Contractual |
Exercise Price |
Options |
Contractual |
||||||||||||||||
|
(CAD$) |
Shares |
Life (Years) |
(CAD$) |
Exercisable |
Life (Years) |
|||||||||||||||
|
$0.10-$0.13 |
1,100,000 | 7.73 | $ | 0.10 | 1,091,667 | 7.74 | ||||||||||||||
|
$0.14-$0.16 |
2,670,000 | 7.92 | 0.15 | 1,780,000 | 7.92 | |||||||||||||||
|
$0.17-$0.26 |
2,689,355 | 8.96 | 0.26 | 1,022,688 | 8.99 | |||||||||||||||
|
$0.27-$0.51 |
2,138,334 | 9.46 | 0.32 | 367,502 | 9.45 | |||||||||||||||
|
$0.52-$1.70 |
1,003,600 | 4.87 | 1.21 | 1,003,000 | 4.87 | |||||||||||||||
| 9,600,689 | 8.21 | $ | 0.32 | 5,264,857 | 7.61 | |||||||||||||||
The cumulative grant date fair value of employee options vested during the years ended December 31, 2017 and 2016 was $63,000 and $122,000, respectively. Total proceeds received for options exercised during the years ended December 31, 2017 and 2016 were $7,000 and $0, respectively.
As of December 31, 2017 and 2016, total compensation expense related to unvested employee stock options not yet recognized was $551,000 and $353,000, respectively, which is expected to be allocated to expenses over a weighted-average period of 1.97 and 2.46 years, respectively.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The assumptions used in calculating the fair value under the Black-Scholes option valuation model are set forth in the following table for options issued by the Company for the years ended December 31, 2017 and 2016:
|
2017 |
2016 |
|||||||
|
Common share fair value |
$0.26 – $0.42 |
$ | 0.16 – 0.24 | |||||
|
Risk-free interest rate |
1.1% | 0.8% | ||||||
|
Expected dividend yield |
0% | 0% | ||||||
|
Expected option life |
4.5 | 4.6 | ||||||
|
Expected stock price volatility |
84.7 – 156.8% | 92.0 – 185.1% | ||||||
Nonemployee share-based compensation
We account for stock options granted to nonemployees in accordance with FASB ASC 505. In connection with stock options granted to nonemployees, we recorded $308,000 and $184,000 for nonemployee share-based compensation during the years ended December 31, 2017 and 2016, respectively. These amounts were based upon the fair values of the vested portion of the grants. Amounts expensed during the remaining vesting period will be determined based on the fair value at the time of vesting.
13. Income Taxes
We have incurred net operating losses since inception. We have not reflected the benefit of net operating loss carryforwards in the accompanying financial statements and have established a full valuation allowance against our deferred tax assets.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes as well as operating losses and tax credit carryforwards.
The significant components of our deferred tax assets and liabilities are as follows (in thousands):
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Deferred tax assets (liabilities): |
||||||||
|
Non-capital losses carried forward |
$ | 7,233 | $ | 6,917 | ||||
|
Research and development expenditures |
887 | 697 | ||||||
|
Share issue costs |
117 | 191 | ||||||
|
Patents and other |
319 | 211 | ||||||
|
Property and equipment |
(4 | ) | 1 | |||||
|
Total deferred tax asset, net |
8,552 | 8,017 | ||||||
|
Valuation allowance |
(8,552 | ) | (8,017 | ) | ||||
|
Net deferred tax asset |
$ | — | $ | — | ||||
Realization of the future tax benefits is dependent on our ability to generate sufficient taxable income within the carry-forward period. Because of our history of operating losses, management believes that the deferred tax assets arising from the above-mentioned future tax benefits are currently not likely to be realized and, accordingly, we have provided a full valuation allowance.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The reconciliation of the Canadian statutory income tax rate applied to the net loss for the year to the income tax expense is as follows:
|
Year Ended December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Statutory income tax rate |
27.0 | % | 27.0 | % | ||||
|
Income tax recovery based on statutory rate |
(1,160 | ) | (594 | ) | ||||
|
Share-based compensation |
110 | 70 | ||||||
|
Gain on revaluation of warrant liability |
(2 | ) | — | |||||
|
Australian research and development incentive |
314 | — | ||||||
|
Share issue costs |
(94 | ) | (88 | ) | ||||
|
Other |
298 | (280 | ) | |||||
|
Change in unrecognized temporary differences |
534 | 892 | ||||||
|
Income tax expense |
— | — | ||||||
Net operating losses and tax credit carryforwards as of December 31, 2017, are as follows:
|
Amount (In thousands) |
Expiration Years |
||||
|
Non-capital income tax losses, net |
$ | 29,943 |
Beginning 2026 |
||
|
Research and development expense carry forwards |
3,284 |
Indefinitely |
|||
|
Tax credits |
525 |
Beginning 2020 |
|||
The Company is subject to taxation in the Canada, the United States and Australia. Tax returns, since the inception of DiaMedica Therapeutics Inc. are subject to examinations by Canadian tax authorities and may change upon examination. Tax returns of DiaMedica USA, Inc., since its inception in 2012 and thereafter, are subject to examination by the U.S. federal and state tax authorities. Tax returns of DiaMedica Therapeutics Australia Pty Ltd., since its inception in 2016 and thereafter, are subject to examination by the Australian tax authorities.
14. Subsequent Events
Sale of common shares and stock purchase warrants
On March 29, 2018, the Company completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiry on March 19, 2020 and March 29, 2020 for Tranche 1 and Tranche 2, respectively. The warrants are subject to early expiry under certain conditions. The warrant expiry date can be accelerated at the option of the Company, in the event that the volume-weighted average trading price of the Company’s common shares exceeds $0.60 per common share for any 21 consecutive trading days. In connection with this offering, the Company paid aggregate finder’s fees of approximately $384,000 and issued an aggregate of 1,610,174 compensation warrants. Each compensation warrant entitles the holder to purchase one common share at $0.245 for a period of 2 years from the closing of this offering, subject to acceleration on the same terms as the common share purchase warrants.
Issuance of common shares on the exercise of stock purchase warrants
During February 2018, 2,425,125 common shares were issued on the exercise of warrants for gross proceeds of approximately $484,000.
Issuance of stock options
On April 17, 2018, the Compensation Committee of the Board of Directors awarded 3,336,000 stock options to various officers, directors and employees of the Company. The options were issued at CAD$0.56 per common share, the closing price of the Company’s common shares on the date of grant and have a ten-year term.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share amounts)
|
March 31, 2018 |
December 31, 2017 |
|||||||
|
(unaudited) |
||||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash |
$ | 6,710 | $ | 1,353 | ||||
|
Amounts receivable |
789 | 80 | ||||||
|
Prepaid expenses |
48 | 61 | ||||||
|
Total current assets |
7,547 | 1,494 | ||||||
|
Deposit |
271 | 271 | ||||||
|
Property and equipment, net |
65 | 37 | ||||||
|
Total non-current assets |
336 | 308 | ||||||
|
Total assets |
$ | 7,883 | $ | 1,802 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable and accrued liabilities |
$ | 1,135 | $ | 919 | ||||
|
Warrant liability |
— | 84 | ||||||
|
Total current liabilities |
1,135 | 1,003 | ||||||
|
Commitments and contingencies |
||||||||
|
Stockholders’ equity: |
||||||||
|
Common shares, no par value; unlimited authorized; 156,297,671 and 127,413,262 shares issued and outstanding, as of March 31, 2018 and December 31, 2017, respectively |
— | — | ||||||
|
Additional paid-in capital |
47,632 | 41,033 | ||||||
|
Accumulated deficit |
(40,884 | ) | (40,234 | ) | ||||
|
Total stockholders’ equity |
6,748 | 799 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 7,883 | $ | 1,802 | ||||
See accompanying notes to the condensed consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
(Unaudited)
|
Three Months Ended March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Operating expenses: |
||||||||
|
Research and development |
$ | 791 | $ | 1,072 | ||||
|
General and administrative |
515 | 283 | ||||||
|
Operating loss |
(1,306 | ) | (1,355 | ) | ||||
|
Other (income) expense: |
||||||||
|
Governmental assistance - research incentives |
(731 | ) | — | |||||
|
Other expense |
35 | 10 | ||||||
|
Change in fair value of warrant liability |
39 | 132 | ||||||
|
Total other (income) expense |
(657 | ) | 142 | |||||
|
Loss before income tax expense |
(649 | ) | (1,497 | ) | ||||
|
Income tax expense |
1 | — | ||||||
|
Net loss and comprehensive loss |
$ | (650 | ) | $ | (1,497 | ) | ||
|
Basic and diluted net loss per share |
$ | (0.01 | ) | $ | (0.01 | ) | ||
|
Weighted average shares outstanding – basic and diluted |
130,935,594 | 110,526,293 | ||||||
See accompanying notes to the condensed consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Stockholders’ Equity
(In thousands except share amounts)
|
Additional |
Total |
|||||||||||||||
|
Common |
Paid-In |
Accumulated |
Stockholders’ |
|||||||||||||
|
Shares |
Capital |
Deficit |
Deficit |
|||||||||||||
|
Balances at December 31, 2017 |
127,413,262 | $ | 41,033 | $ | (40,234 | ) | $ | 799 | ||||||||
|
Issuance of common shares and warrants, net of offering costs of $529 |
26,459,284 | 5,840 | — | 5,840 | ||||||||||||
|
Exercise of common share purchase warrants |
2,425,125 | 606 | — | 606 | ||||||||||||
|
Share-based compensation expense |
— | 153 | — | 153 | ||||||||||||
|
Net loss |
— | — | (650 | ) | (650 | ) | ||||||||||
|
Balances at March 31, 2018 |
156,297,671 | $ | 47,632 | $ | (40,884 | ) | $ | 6,748 | ||||||||
See accompanying notes to the condensed consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
|
Three Months Ended March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (650 | ) | $ | (1,497 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Share-based compensation |
153 | 97 | ||||||
|
Change in fair value of warrant liability |
39 | 132 | ||||||
|
Depreciation |
4 | 1 | ||||||
|
Changes in operating assets and liabilities: |
||||||||
|
Amounts receivable |
(709 | ) | 1 | |||||
|
Prepaid expenses |
13 | 13 | ||||||
|
Accounts payable and accrued liabilities |
215 | 436 | ||||||
|
Net cash used in operating activities |
(935 | ) | (817 | ) | ||||
|
Cash flows from investing activities: |
||||||||
|
Purchase of property and equipment |
(32 | ) | — | |||||
|
Net cash used in financing activities |
(32 | ) | — | |||||
|
Cash flows from financing activities: |
||||||||
|
Proceeds from issuance of common shares and warrants, net of offering costs |
5,840 | — | ||||||
|
Proceeds from the exercise of common share purchase warrants |
484 | — | ||||||
|
Proceeds from the exercise of stock options |
— | 7 | ||||||
|
Net cash provided by financing activities |
6,324 | 7 | ||||||
|
Net increase (decrease) in cash |
5,357 | (810 | ) | |||||
|
Cash at beginning of year |
1,353 | 1,736 | ||||||
|
Cash at end of year |
$ | 6,710 | $ | 926 | ||||
See accompanying notes to the condensed consolidated financial statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
DiaMedica Therapeutics Inc.
Notes to the Condensed Consolidated Financial Statements
1. Business
DiaMedica Therapeutics Inc. and its wholly-owned subsidiaries DiaMedica USA, Inc. and DiaMedica Australia Pty Ltd. (collectively “we,” “us,” “our” and the “Company”) exist for the primary purpose of advancing the clinical and commercial development of a proprietary recombinant KLK1 protein for the treatment of acute ischemic stroke and chronic kidney disease. The Company is a listed company incorporated under the Canada Business Corporations Act and domiciled in British Columbia, Canada, whose shares are publicly traded on the TSX Venture Exchange in Canada under the symbol “DMA” and the OTCQB in the United States under the symbol “DMCAF”.
2. Risks and Uncertainties
The Company operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (“FDA”) in the United States, the Therapeutic Goods Administration (“TGA”) in Australia, the European Medicines Agency (“EMA”) in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditures.
As of March 31, 2018, we have incurred losses of $40.9 million since our inception in 2000. For the three months ended March 31, 2018, we incurred a net loss of $650,000, and incurred negative cash flows from operating activities of $935,000 for this period. We expect to incur substantial losses for the foreseeable future, which will continue to generate negative net cash flows from operating activities, as we continue to pursue research and development activities and the clinical development of our primary product candidate, DM199. As of March 31, 2018, we had cash of $6.7 million, working capital of $6.4 million and stockholders’ equity of $6.7 million. The Company’s principal sources of cash have included the issuance of equity securities.
The accompanying Consolidated Financial Statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our initial product candidate, DM199, in the United States, Australia, the European Union or other markets and ultimately our ability to market and sell our initial product candidate. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See Note 3 titled “Liquidity, Management’s Plans and Going Concern.”
3. Liquidity, Management Plans and Going Concern
As of December 31, 2017 and March 31, 2018, the Company has an accumulated deficit of $40.2 million and $40.9 million, respectively, and the Company has not generated positive cash flow from operations since its inception.
Additional funding will be required to continue the Company’s research and development and other operating activities. In the next 12 months we will likely seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data is not positive or economic and market conditions deteriorate.
During March 2018, the Company completed a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. In addition, during February 2018, 2,425,125 common shares were issued on the exercise of warrants for gross proceeds of approximately $484,000.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
If we are unable to obtain additional financing when needed, we would need to scale back our operations taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modify or delay the development of our DM199 product candidate, license to third parties the rights to commercialize our DM199 product candidate for acute ischemic stroke, chronic kidney disease or other applications that we would otherwise seek to pursue, or cease operations.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to demonstrate clinical progress for our DM199 product candidate in the United States or other markets, our ability to obtain required governmental approvals of our product candidate and ultimately our ability to license or market and sell our DM199 product candidate. If we are unable to obtain additional financing when needed, if our clinical trials are not successful, if we are unable to obtain required governmental approvals, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional equity securities would likely result in dilution to our current stockholders.
4. Basis of presentation
We have prepared the accompanying interim condensed Consolidated Financial Statements in accordance with accounting principles general accepted in the United States (“US GAAP”) for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the Securities and Exchange Commission (“SEC”). Accordingly, they do not include all of the information and footnotes required by US GAAP for complete financial statements. These interim condensed Consolidated Financial Statements reflect all adjustments consisting of normal recurring accruals, which, in the opinion of management, are necessary to present fairly our consolidated financial position, consolidated results of operations, consolidated statement of stockholders’ equity and consolidated cash flows for the periods and as of the dates presented. Our fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2017 was derived from audited Consolidated Financial Statements but does not include all disclosures required by U.S. GAAP. These interim condensed Consolidated Financial Statements should be read in conjunction with the annual Consolidated Financial Statements and the notes thereto. The nature of our business is such that the results of any interim period may not be indicative of the results to be expected for the entire year. Certain prior year amounts have been reclassified to conform to the current basis of presentation.
Recently issued accounting pronouncement
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases. The guidance in ASU 2016-02 supersedes the lease recognition requirements in the Accounting Standards Codification Topic 840, Leases. ASU 2016-02 requires an entity to recognize assets and liabilities arising from a lease for both financing and operating leases, along with additional qualitative and quantitative disclosures. The new standard requires the immediate recognition of all excess tax benefits and deficiencies in the income statement and requires classification of excess tax benefits as an operating activity as opposed to a financing activity in the statements of cash flows. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, with early adoption permitted. Management is evaluating the standard's impact on the Consolidated Financial Statements.
In June 2018, the FASB issued ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which simplifies several aspects of the accounting for nonemployee share-based payment transactions resulting from expanding the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. This ASU is effective for the Company for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. The Company is currently evaluating the impact of the new guidance on our Consolidated Financial Statements.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
5. Summary of Significant Accounting Policies
Principles of consolidation
The accompanying condensed Consolidated Financial Statements include the assets, liabilities and expenses of DiaMedica Therapeutics Inc., and our wholly-owned subsidiaries DiaMedica USA, Inc. and DiaMedica Australia Pty Ltd. All significant intercompany transactions and balances have been eliminated in consolidation.
Functional currency
The United States dollar is the functional currency that represents the economic effects of the underlying transactions, events and conditions and various other factors including the currency of historical and future expenditures and the currency in which funds from financing activities are mostly generated by the Company. A change in the functional currency occurs only when there is a material change in the underlying transactions, events and condition. A change in functional currency could result in material differences in the amounts recorded in the consolidated statement of loss and comprehensive loss for foreign exchange gains and losses. All amounts in the accompanying Consolidated Financial Statements are in U.S. dollars unless otherwise indicated.
Use of estimates
The preparation of Consolidated Financial Statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentration of credit risk
Financial instruments that potentially subject the company to significant concentrations of credit risk consist primarily of cash. Cash is deposited in demand accounts at commercial banks. At times, such deposits may be in excess of insured limits. The Company has not experienced any losses on its deposits of cash.
Fair value of financial instruments
Carrying amounts of certain of the Company’s financial instruments, including amounts receivable, prepaid expenses and other current assets, accounts payable and accrued liabilities approximate fair value due to their short maturities. Certain of the Company’s common share purchase warrants are required to be reported at fair value. The fair value of common share purchase warrants is disclosed in Note 9 titled “Warrant Liability.”
Fair value measurements
Fair value is defined as the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The hierarchy is broken down into three levels defined as follows:
|
● |
Level 1—Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
|
● |
Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active or model-derived valuations for which all significant inputs are observable, either directly or indirectly. |
|
● |
Level 3—Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable. |
Our cash and equivalents consist of bank deposits. As of March 31, 2018, the Company believes that the carrying amounts of its other financial instruments, including amounts receivable, accounts payable and accrued liabilities, approximate their fair value due to the short-term maturities of these instruments.
Common share warrant liability
The common share warrants that were issued in connection with the February 2016 private placements of common shares are classified as a liability in the consolidated balance sheets, as the common share warrants have an exercise price stated in Canadian dollars, which is different than the functional currency, and thus these warrants qualify as a derivative instruments. The fair value of these common share warrants is re-measured at each financial reporting period and immediately before exercise, with any changes in fair value being recognized as a component of other income (expense) in the consolidated statements of operations. These warrants were exercised in February 2018, see Note 9 titled “Warrant Liability.”
Long-lived assets
Property and equipment are stated at purchased cost less accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of three to ten years for office equipment and four years for computer equipment. Upon retirement or sale, the cost and related accumulated depreciation are removed from the consolidated balance sheets and the resulting gain or loss is reflected in the consolidated statements of operations. Repairs and maintenance are expensed as incurred.
Long-lived assets are evaluated for impairment when events or changes in circumstances indicate that the carrying amount of the asset or related group of assets may not be recoverable. If the expected future undiscounted cash flows are less than the carrying amount of the asset, an impairment loss is recognized at that time. Measurement of impairment may be based upon appraisal, market value of similar assets or discounted cash flows.
Research and development costs
Research and development costs include expenses incurred in the conduct of human clinical trials, for third-party service providers performing various testing and accumulating data related to non-clinical studies; sponsored research agreements; developing the manufacturing process necessary to produce sufficient amounts of the DM199 compound for use in our non-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our DM199 product candidate; and personnel costs, including salaries, benefits and share-based compensation.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual work performed at each clinical trial site and by each CRO.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Share-based compensation
The cost of employee and non-employee services received in exchange for awards of equity instruments is measured and recognized based on the grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the vesting period, which is considered to be the requisite service period. We record forfeitures in the periods in which they occur.
The fair value of share-based awards is estimated at the date of grant using the Black-Scholes option pricing model. The determination of the fair value of share-based awards is affected by our stock price, as well as assumptions regarding a number of complex and subjective variables. Risk free interest rates are based upon Canadian Government bond rates appropriate for the expected term of each award. Expected volatility rates are based on the on historical volatility equal to the expected life of the option. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past.
Government assistance
Government assistance relating to research and development performed by DiaMedica Australia Pty Ltd. is recorded as a component of Other Income (Expense). Government assistance is initially recognized when reasonable assurance exists that the Company will comply with the conditions attached to the incentive program and that the incentive payments will be received. In subsequent periods, the government assistance is recognized when the related expenditures are incurred.
Net loss per share
We compute net loss per share by dividing our net loss (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period, if any, are weighted for the portion of the period that they were outstanding. The computation of diluted earnings per share, or EPS, is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Our diluted EPS is the same as basic EPS due to common equivalent shares being excluded from the calculation, as their effect is anti-dilutive.
The following table summarizes our calculation of net loss per common share for the periods (in thousands, except share and per share data):
|
Three Months Ended March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Net loss |
$ | (650 | ) | $ | (1,497 | ) | ||
|
Weighted average shares outstanding—basic and diluted |
130,935,594 | 110,526,293 | ||||||
|
Basic and diluted net loss per share |
$ | (0.01 | ) | $ | (0.01 | ) | ||
The following outstanding potential common shares were not included in the diluted net loss per share calculations as their effects were not dilutive:
|
Three Months Ended March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Employee and non-employee stock options |
$ | 9,600,689 | $ | 7,461,334 | ||||
|
Common shares issuable under common share purchase warrants |
16,652,026 | 2,562,050 | ||||||
|
Common shares issuable under deferred share unit plan |
423,676 | 423,676 | ||||||
| $ | 26,676,391 | $ | 10,447,060 | |||||
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
6. Amounts Receivable
Amounts receivable consisted of the following (in thousands):
|
March 31, 2018 |
December 31, 2017 |
|||||||
|
Research and development incentives |
$ | 731 | $ | — | ||||
|
Sales-based taxes receivable |
58 | 80 | ||||||
|
Total amounts receivable |
$ | 789 | $ | 80 | ||||
7. Deposit
Deposit consisted of the following (in thousands):
|
March 31, 2018 |
December 31, 2017 |
|||||||
|
Advances to vendor |
$ | 271 | $ | 271 | ||||
|
Total deposit |
$ | 271 | $ | 271 | ||||
We have advanced funds to a vendor engaged to support the performance of the REMEDY Phase 2 clinical trial. The funds advanced will be held, interest free, by this vendor until the completion of the trial and applied to final trial invoices or refunded. This deposit is classified as non-current as the trial is not expected to be completed during 2018
8. Property and Equipment
Property and equipment consisted of the following (in thousands):
|
March 31, 2018 |
December 31, 2017 |
|||||||
|
Furniture and equipment |
$ | 53 | $ | 40 | ||||
|
Computer equipment |
42 | 23 | ||||||
| 95 | 63 | |||||||
|
Less accumulated depreciation |
(30 | ) | (26 | ) | ||||
|
Property and equipment, net |
$ | 65 | $ | 37 | ||||
9. Accrued Liabilities
Accounts payable and accrued liabilities consisted of the following (in thousands):
|
March 31, 2018 |
December 31, 2017 |
|||||||
|
Trade and other payables |
$ | 483 | $ | 513 | ||||
|
Accrued compensation and related |
409 | 356 | ||||||
|
Offering costs |
186 | — | ||||||
|
Accrued research and other professional fees |
45 | 45 | ||||||
|
Other accrued liabilities |
12 | 5 | ||||||
|
Total accrued liabilities |
$ | 1,135 | $ | 919 | ||||
10. Warrant Liability
In February 2016, the Company completed, in two tranches, a non-brokered private placement of 4,687,500 units with each unit consisting of one common share and one half of one common share purchase warrant. The Company issued 2,343,750 warrants. Each warrant entitles the holder to purchase one common share at a price of $0.25 Canadian dollars at any time prior to expiry on February 18 or 25, 2018 for Tranche 1 and Tranche 2, respectively.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
As the warrant exercise price is stated in Canadian dollars and the Company’s functional currency is the U.S. dollar, the warrants are deemed to be a derivative, with their estimated fair value classified as a liability on the Company’s balance sheet. The initial estimated fair value of the warrants was recorded as a warrant liability with subsequent changes in the estimated fair value recognized in the Consolidated Statements of Operations and Comprehensive Loss. The Company allocated $281,000 of the net proceeds to the warrant liability and the balance of the proceeds to the common shares (Note 9). The initial fair value of the warrants was determined using a Black-Scholes pricing model with the following assumptions: expected volatilities of 191.8 – 225.0%, risk-free interest rates of 0.43 – 0.49%, and expected life of 2 years.
In connection with the offering, the Company issued an aggregate of 218,300 compensation warrants. Each compensation warrant entitles the holder to purchase one common share at $0.25 Canadian dollars for a period of 2 years from the date of issuance, subject to acceleration on the same terms as the common share purchase warrants. The Company estimated the value of these warrants at $24,000, which was included in the issuance costs. The initial fair value of the warrants was determined using a Black-Scholes pricing model with the following assumptions: expected volatilities of 191.8 – 225.0%, risk-free interest rates of 0.43 – 0.49%, and expected life of 2 years.
During February 2018, 2,425,125 common shares were issued on the exercise of warrants for gross proceeds of approximately $483,000 and the remaining 86,925 warrants expired.
The fair value of the Company’s common share purchase warrant liability, for both investor warrants and compensation warrants, is calculated using a Black-Scholes valuation model and is classified as Level 3 in the fair value hierarchy. The fair values at the time of exercise of the warrants were estimated using the following valuation assumptions:
|
Warrant Valuation |
|
|
Common share fair value |
$0.31 |
|
Risk-free interest rate |
1.84% |
|
Expected dividend yield |
0% |
|
Expected life (years) |
0.01 – 0.03 |
|
Expected stock price volatility |
16.7% |
The following is a rollforward of the fair value of Level 3 warrants (in thousands):
|
Warrant Liability |
||||
|
Ending balance December 31, 2017 |
$ | 84 | ||
|
Change in fair value |
39 | |||
|
Exercises |
(123 | ) | ||
|
Ending balance March 31, 2018 |
$ | — | ||
11. Stockholders’ Deficit
Authorized capital stock
The Company has authorized share capital of an unlimited number of common voting shares and the shares have no stated value.
Common shareholders are entitled to receive dividends as declared by the Company, if any, and are entitled to one vote per share at the Company's annual general meeting and any extraordinary general meeting.
Private placements during 2018
On March 29, 2018, the Company completed, in two tranches, a brokered and non-brokered private placement of 26,489,284 units at a price of $0.245 per unit for aggregate gross proceeds of approximately $6.3 million. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiry on March 19, 2020 and March 29, 2020 for Tranche 1 and Tranche 2, respectively. The warrants are subject to early expiry under certain conditions. The warrant expiry date can be accelerated at the option of the Company, in the event that the volume-weighted average trading price of the Company’s common shares exceeds $0.60 per common share for any 21 consecutive trading days. In connection with this offering, the Company paid aggregate finder’s fees of approximately $384,000 and issued an aggregate of 1,610,174 compensation warrants. Each compensation warrant entitles the holder to purchase one common share at $0.245 for a period of 2 years from the closing of this offering, subject to acceleration on the same terms as the common share purchase warrants.
During the three months ended March 31, 2018, 2,425,125 common shares were issued on the exercise of warrants for gross proceeds of $484,000.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Private placements during 2017
On December 18, 2017, the Company completed a non-brokered private placement of 3,624,408 units at a price of $0.26 per unit for aggregate gross proceeds of approximately $944,000, or $934,000 net of issuance costs. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.35 at any time prior to expiry on December 19, 2019. Warrants are subject to early expiry, at the option of the Company, if on any date the volume-weighted average closing trading price of the common shares on any recognized Canadian stock exchange equals or exceeds $0.60 for a period of 21 consecutive trading days.
On April 17, 2017, the Company completed a non-brokered private placement of 10,526,315 units at a price of $0.19 per unit for aggregate gross proceeds of approximately $2,000,000, or $1,983,000 net of issuance costs. Each unit consisted of one common share and one half of one common share purchase warrant. Each whole warrant entitles the holder to purchase one common share at a price of $0.23 at any time prior to expiry on April 17, 2019. Warrants are subject to early expiry, at the option of the Company, if on any date the volume-weighted average closing trading price of the common shares on any recognized Canadian stock exchange equals or exceeds $0.30 for a period of 10 consecutive trading days.
During the year ended December 31, 2017, 50,000 common shares were issued on the exercise of warrants for gross proceeds of $9,913 and 60,000 common shares were issued on the exercise of options for gross proceeds of $6,749.
Shares reserved
Shares of common stock reserved for future issuance are as follows:
|
March 31, 2018 |
||||
|
Stock options outstanding |
9,600,689 | |||
|
Deferred share units outstanding |
423,676 | |||
|
Shares available for grant under the DiaMedica Stock Option Plan |
6,029,078 | |||
|
Common shares issuable under common stock purchase warrants |
16,652,026 | |||
|
Total |
32,705,469 | |||
12. Share-based Compensation
Deferred share unit plan
The 2012 Deferred Share Unit Plan (the “2012 DSU Plan”) promotes greater alignment of long-term interests between non-executive directors and executive officers of the Company and its shareholders through the issuance of deferred share units (“DSUs”). Since the value of DSUs increases or decreases with the market price of the common shares, DSUs reflect a philosophy of aligning the interests of directors and executive officers by tying compensation to share price performance. For the three months ended March 31, 2018 and 2017, there were no shares issued. The Company has reserved for issuance up to 2,000,000 common shares under the 2012 DSU Plan and 423,676 DSUs were outstanding at March 31, 2018.
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
Stock option plan
DiaMedica has adopted a Stock Option Plan (the “Option Plan”) where the Board of Directors may from time to time, in their sole discretion, and in accordance with the requirements of the Toronto (TSX) Venture Exchange, grant to directors, officers, management company employees, investor relations consultants and Consultants (as such terms are used in the Stock Option Plan) to DiaMedica, non-transferable options to purchase common shares. The shareholders approved the adoption of a Option Plan on September 22, 2011, and as amended and restated on October 23, 2015 and December 21, 2017, reserving for issuance up to 10% of the Company’s issued and outstanding common shares. Options granted vest at various rates and have terms of up to 10 years. As of March 31, 2018, options to purchase 9,600,689 common shares were outstanding. As the TSX Venture Exchange is the principle trading market for the Company’s shares, all options have been priced in Canadian dollars.
The aggregate number of common shares reserved as of March 31, 2018 was 15,629,767, which includes both the Option Plan and the 2012 DSU Plan.
Share-based compensation expense for each of the periods presented is as follows (in thousands):
|
Three Months Ended |
||||||||
|
March 31, 2018 |
March 31, 2017 |
|||||||
|
Research and development |
$ | 22 | $ | 9 | ||||
|
General and administrative |
131 | 88 | ||||||
|
Total share-based compensation |
$ | 153 | $ | 97 | ||||
We recognize share-based compensation based on the fair value of each award as estimated using the Black-Scholes option valuation model. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
A summary of option activity is as follows:
|
Shares Underlying Options |
Weighted Average Exercise Price Per Share (CAD$) |
Aggregate Intrinsic Value (CAD$) |
||||||||||
|
Balances at December 31, 2017 |
9,600,689 | $ | 0.32 | $ | 674,000 | |||||||
|
Granted |
— | — | ||||||||||
|
Exercised |
— | — | ||||||||||
|
Expired / cancelled |
— | — | ||||||||||
|
Forfeited |
— | — | ||||||||||
|
Balances at March 31, 2018 |
9,600,689 | $ | 0.32 | $ | 1,901,000 | |||||||
Information about stock options outstanding, vested and expected to vest as of March 31, 2018, is as follows:
|
Outstanding, Vested and Expected to Vest |
Options Vested and Exercisable |
|||||||||||||||||||
|
Weighted Average |
Weighted |
Weighted Average |
||||||||||||||||||
|
Per Share |
Remaining |
Average |
Remaining |
|||||||||||||||||
|
Exercise Price |
Contractual |
Exercise Price |
Options |
Contractual |
||||||||||||||||
|
(CAD$) |
Shares |
Life (Years) |
(CAD$) |
Exercisable |
Life (Years) |
|||||||||||||||
|
$0.10-$0.13 |
1,100,000 | 7.48 | $ | 0.10 | 1,100,000 | 7.48 | ||||||||||||||
|
$0.14-$0.16 |
2,670,000 | 7.67 | 0.15 | 2,002,500 | 7.67 | |||||||||||||||
|
$0.17-$0.26 |
2,689,355 | 8.71 | 0.26 | 1,295,606 | 8.75 | |||||||||||||||
|
$0.27-$0.51 |
2,138,334 | 9.21 | 0.32 | 544,584 | 9.21 | |||||||||||||||
|
$0.52-$1.70 |
1,003,000 | 4.62 | 1.21 | 1,003,000 | 4.62 | |||||||||||||||
| 9,600,689 | 7.97 | $ | 0.32 | 5,945,690 | 7.50 | |||||||||||||||
CONFIDENTIAL TREATMENT REQUESTED
BY DIAMEDICA THERAPEUTICS INC.
PURSUANT TO 17 C.F.R. SECTION 200.83
The cumulative grant date fair value of employee options vested during the three months ended March 31, 2018 and 2017 was $418,000 and $278,000, respectively. Total proceeds received for options exercised during the three months ended March 31, 2018 and 2017 were $0 and $7,000, respectively.
Nonemployee share-based compensation
We account for stock options granted to nonemployees in accordance with FASB ASC 505. In connection with stock options granted to nonemployees, we recorded $125,000 and $93,000 for nonemployee share-based compensation during the three months ended March 31, 2018 and 2017, respectively. These amounts were based upon the fair values of the vested portion of the grants. Amounts expensed during the remaining vesting period will be determined based on the fair value at the time of vesting.
F-34