
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-235775
PROSPECTUS SUPPLEMENT
(To Prospectus dated January 9, 2020)

2,125,000 Common Shares
We are offering 2,125,000 common shares, no par value per share. Our common shares are listed on The Nasdaq Capital Market under the symbol “DMAC.” On February 10, 2020, the closing price of our common shares as reported on The Nasdaq Capital Market was $4.68 per share.
We are an “emerging growth company” and a “smaller reporting company” as defined under federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements. See “Prospectus Supplement Summary – Our Company – Implications of Being an Emerging Growth Company”.
Investing in our common shares involves a high degree of risk. You should carefully consider the matters set forth in “Risk Factors” beginning on page S-10 of this prospectus supplement, page 4 of the accompanying prospectus and under similar headings in the documents that are incorporated by reference into this prospectus supplement.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus supplement. Any representation to the contrary is a criminal offense.
|
Per Share |
Total |
|||||||
|
Public offering price |
$ | 4.00 | $ | 8,500,000 | ||||
|
Underwriting discounts and commissions(1) |
$ | 0.28 | $ | 595,000 | ||||
|
Proceeds, before expenses, to us |
$ | 3.72 | $ | 7,905,000 | ||||
|
(1) |
In addition to the underwriting discount, we have agreed to reimburse the underwriter for certain expenses. See “Underwriting” for additional information regarding underwriting compensation. |
The underwriter expects to deliver the shares against payment through the facilities of the Depository Trust Company on or about February 13, 2020, subject to customary closing conditions.
Craig-Hallum Capital Group
The date of this prospectus supplement is February 11, 2020
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
|
ABOUT THIS PROSPECTUS SUPPLEMENT |
S-1 |
|
INDUSTRY AND MARKET DATA |
S-1 |
|
PROSPECTUS SUPPLEMENT SUMMARY |
S-2 |
|
RISK FACTORS |
S-10 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
S-45 |
|
USE OF PROCEEDS |
S-46 |
|
DIVIDEND POLICY |
S-47 |
|
CAPITALIZATION |
S-48 |
|
DILUTION |
S-49 |
|
DESCRIPTION OF COMMON SHARES |
S-50 |
|
CERTAIN UNITED STATES INCOME TAX CONSIDERATIONS |
S-61 |
|
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS |
S-68 |
|
UNDERWRITING |
S-70 |
|
LEGAL MATTERS |
S-75 |
|
EXPERTS |
S-75 |
|
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE |
S-75 |
|
WHERE YOU CAN FIND MORE INFORMATION |
S-76 |
| BASE PROSPECTUS | |
|
ABOUT THIS PROSPECTUS |
1 |
|
ABOUT THE COMPANY |
2 |
|
RISK FACTORS |
4 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
4 |
|
USE OF PROCEEDS |
5 |
|
DILUTION |
5 |
|
DESCRIPTION OF OUR COMMON SHARES |
6 |
|
DESCRIPTION OF WARRANTS |
11 |
|
DESCRIPTION OF UNITS |
12 |
|
PLAN OF DISTRIBUTION |
13 |
|
LEGAL MATTERS |
14 |
|
EXPERTS |
14 |
|
WHERE YOU CAN FIND MORE INFORMATION |
15 |
|
INCORPORATION OF DOCUMENTS BY REFERENCE |
15 |
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus are part of a registration statement that we filed with the United States Securities and Exchange Commission (SEC) utilizing a “shelf” registration process on Form S-3 (File No. 333-235775) that was declared effective by the SEC on January 9, 2020. This prospectus supplement describes the specific details regarding this offering, including the price, the amount of our common shares being offered, certain risks of investing in our common shares and other items. You should read this entire prospectus supplement, as well as the accompanying prospectus, together with the additional information described under “Incorporation of Certain Documents by Reference” and “Where You Can Find More Information” carefully before making an investment decision. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus or in any document incorporated by reference that was filed with the SEC before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date – for example, a document incorporated by reference in the accompanying prospectus – the statement in the document having the later date modifies or supersedes the earlier statement. You should assume that the information contained in this prospectus supplement is accurate as of the date on the front cover of this prospectus supplement only and that any information we have incorporated by reference or included in the accompanying prospectus is accurate only as of the date given in the document incorporated by reference or as of the date of the prospectus, as applicable, regardless of the time of delivery of this prospectus supplement or the accompanying prospectus or any sale of our common shares. Our business, financial condition, liquidity, results of operations and prospects may have changed since that date.
All references in this prospectus supplement to “$,” “U.S. Dollars” and “dollars” are to United States dollars.
We own various unregistered trademarks and service marks, including our corporate logo. Solely for convenience, the trademarks and trade names in this prospectus supplement are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the owner of such trademarks and trade names will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
INDUSTRY AND MARKET DATA
In addition to the industry, market and competitive position data referenced in this prospectus supplement from our own internal estimates and research, some market data and other statistical information included in this prospectus supplement are based in part upon information obtained from third-party industry publications, research, surveys and studies, none of which we commissioned. Third-party industry publications, research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
We are responsible for all of the disclosure in this prospectus supplement and while we believe that each of the publications, research, surveys and studies included in this prospectus supplement are prepared by reputable sources, neither we nor the underwriter has independently verified market and industry data from third-party sources. In addition, while we believe our internal company research and estimates are reliable, such research and estimates have not been verified by independent sources. Assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Cautionary Note Regarding Forward-Looking Statements.”
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights information contained in this prospectus supplement, or incorporated by reference into this prospectus supplement, and does not contain all of the information that you should consider in making your investment decision. Before investing in our common shares, you should carefully read this entire prospectus supplement, including the information set forth under “Risk Factors,” and our financial statements and the related notes thereto, in each case included in this prospectus supplement or incorporated by reference into this prospectus supplement. Some of the statements in this prospectus supplement constitute forward‑looking statements. See “Cautionary Note Regarding Forward‑Looking Information.”
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus supplement to “DiaMedica,” “the Company,” “we,” “us,” “our” or similar references mean DiaMedica Therapeutics Inc. and its subsidiaries. References in this prospectus supplement to “voting common shares” or “common shares” mean our voting common shares, no par value per share.
Our Company
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant (synthetic) proteins. Our goal is to use our trade secrets and patented and licensed technologies to establish our company as a leader in the development and commercialization of therapeutic treatments derived from novel recombinant proteins. Our current focus is on chronic kidney disease (CKD) and acute ischemic stroke (AIS). We plan to advance DM199, our lead drug candidate, through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for CKD and AIS.
DM199 is a recombinant form of human tissue kallikrein-1 (KLK1). KLK1 is a serine protease (protein) produced primarily in the kidneys, pancreas and salivary glands that plays a critical role in the regulation of local blood flow and vasodilation (the widening of blood vessels, which decreases vascular resistance) in the body, as well as an important role in inflammation and oxidative stress (an imbalance between potentially damaging reactive oxygen species, or free radicals, and antioxidants in your body). We believe DM199 has the potential to treat a variety of diseases where healthy functioning requires sufficient activity of KLK1 and its system, the kallikrein-kinin system (KKS).
CKD and AIS patients suffer from impaired blood flow to the kidneys and brain, respectively. These patients also tend to exhibit lower than normal levels of endogenous (produced by the body) KLK1. We believe treatment with DM199 could replenish levels of KLK1, thereby releasing physiological levels of bradykinin (BK) when and where needed, generating beneficial nitric oxide and prostacyclin, setting in motion metabolic pathways that can improve blood flow (through vasoregulation), dampen inflammation, and protect tissues and end-organs from ischemic damage, supporting structural integrity and normal functioning.
Today, forms of KLK1 derived from human urine and porcine pancreas are sold in Japan, China and Korea to treat AIS, CKD, retinopathy, hypertension and related vascular diseases. We believe millions of patients have been treated with these KLK1 therapies, and the data from more than 100 published papers and studies support their clinical benefit. However, there are numerous regulatory, commercial, and clinical drawbacks associated with KLK1 derived from human urine and porcine pancreas that can be overcome by developing a synthetic version of KLK1 such as DM199. We believe regulatory drawbacks are the primary reason why KLK1 derived from human urine and porcine pancreas are not currently available and used in the United State or Europe. We are not aware of any synthetic version of KLK1 with regulatory approval for human use in any country, nor are we aware of any synthetic version in development other than our drug candidate, DM199.
We have conducted numerous internal and third-party analyses to evaluate the structural and functional performance of DM199 as compared to KLK1 derived from human urine. The results of these studies have demonstrated that DM199 is structurally and functionally equivalent to KLK1 derived from human urine in that (i) the amino acid structure of DM199 is identical to the human urine form, (ii) the enzymatic and pharmacokinetic profiles are substantially similar to human urinary derived KLK1 and (iii) the physiological effects of DM199 on blood pressure mirror that of human urinary derived KLK1. We believe that the results of this work suggest that the therapeutic action of DM199 will be the same or better than that of the forms of KLK1 marketed in Asia. In addition, we have completed enrollment in seven clinical trials with DM199 treating over 240 volunteers, and the results have shown that DM199 has been well-tolerated. However, DM199 has not been, and we cannot provide any assurance that it ultimately will be, determined to be safe or effective for purposes of granting marketing approval by the FDA or any comparable agency.
Our recombinant form of DM199 is protected by issued composition of matter and delivery patents in the United States and Europe (expiration 2033); a pending worldwide patent (expiration 2038) that covers a range of DM199 dose levels and dosing regimens useful for treating a wide range of diseases associated with microvascular dysfunction; an exclusive license with our manufacturing partner for use of their cell line and proprietary expression system for manufacturing synthetic KLK1; and numerous trade-secrets. In addition, we believe DM199 cannot be reverse engineered to develop a copycat version of our therapy. This adds additional protection to our intellectual property, especially as we engage in DM199 licensing activities.
Our Programs
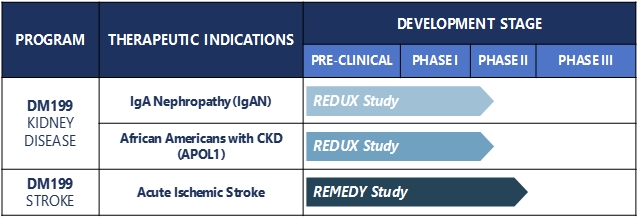
The primary focus for our DM199 program development is on CKD and AIS. The current status of our product candidates in clinical development is as follows:
|
● |
Chronic Kidney Disease. CKD is a widespread health problem that generates significant economic burden throughout the world. According to the National Kidney Foundation, 30 million Americans and 120 million Chinese suffer from this debilitating and potentially life-threatening condition. CKD is characterized by a progressive decline in overall kidney function, increasing the risk of premature death, cardiovascular events and hospitalization. End stage renal disease (ESRD) is the final stage of CKD and requires ongoing dialysis or a kidney transplant to survive, but many patients suffer serious health consequences or die from CKD prior to developing ESRD. Currently, there is no cure for CKD and treatment involves management of the symptoms of the disease. Blood pressure medications, such as angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), are often prescribed to control hypertension, and hopefully, slow the progression of CKD. Nevertheless, according to the National Kidney Foundation, many of these patients continue to show declining kidney function. We believe DM199 offers a potentially novel approach for the treatment of CKD because KLK1 protein plays a vital role in normal kidney function. Since patients with moderate to severe CKD often excrete abnormally low levels of KLK1 in their urine, we believe that DM199, by increasing levels of KLK1 and restoring the protective kallikrein-kinin system to regulate the production and release of nitric oxide and prostacyclin, may prevent or reduce further kidney damage. |
|
● |
Acute Ischemic Stroke. According to the World Health Organization, each year approximately 15 million people worldwide suffer a stroke, of which 5.0 million will die and 5.0 million will be permanently disabled. According to the U.S. Center for Disease Control and Prevention is approximately 87% of all strokes are ischemic in nature. We believe that stroke represents an area of significant unmet medical need, and a KLK1 treatment (such as DM199) could provide a significant patient benefit with its proposed therapeutic window of up to 24 hours after the first sign of symptoms. Currently, the only FDA-approved pharmacological intervention for AIS is tissue plasminogen activator (tPA), must be given within 4.5 hours of symptom onset. Treating patients with tPA during this time window can be challenging because it is difficult to determine precisely when symptoms began and a patient must undergo complex brain imaging before treatment to rule out a hemorrhagic stroke. Mechanical thrombectomy, a procedure in which the clot is removed using catheter-based tools, is also available to certain patients. Despite the availability of these treatments, we believe they are relevant to less than 10% of ischemic stroke patients due to the location of the clot, the elapsed time after the stroke occurred, or safety considerations. Thus, we believe DM199 may offer significant advantages over the current treatment options and fill an unmet need for patients who cannot receive tPA or mechanical thrombectomy. Additionally, DM199 may also offer a complimentary follow-on treatment for patients who initially receive tPA or mechanical thrombectomy treatments by enabling sustained blood flow improvements to the brain during the critical first few weeks after a stroke. Based on the number of strokes each year (approximately 1.7 million in the U.S., Europe and Japan and 15 million worldwide) and considering the $8,500 estimated cost per patient for the current standard of care, tPA, we believe the annual market opportunity for DM199 could be significant. |
Our Clinical Trials
In July 2019, we completed a Phase Ib clinical trial of DM199 in participants with moderate or severe CKD caused by Type I or Type II diabetes. We initiated dosing patients in this study in February 2019 The study was performed to assess the pharmacokinetics (PK) of three dose levels of DM199 (3, 5 and 8 µg/kg), administered in a single subcutaneous dose, as well as the evaluation of safety, tolerability and secondary pharmacodynamic (PD) endpoints. The study results demonstrated that at the 3µg/kg dose level, the PK profiles were similar between moderate and severe CKD patients, and consistent with healthy subjects (normal kidney function) tested previously, and that DM199 was well tolerated with no dose-limiting tolerability. There were no deaths, no discontinuations due to a treatment-related adverse event (AE), and no treatment-related significant adverse events (SAEs). AEs were minor and consistent with standard treatment(s) in the CKD patient population. We announced favorable overall interim PD results from the first 28 subjects that included short-term improvements in Nitric Oxide (NO), average increase of 35.2%, Prostaglandin E2 (PGE2), average increase of 41.2%, estimated glomerular flow rate (eGFR), average increase of 4.08 mL/min/1732, and the urinary albumin to creatinine ratio (UACR) excluding subjects with normal UACR levels, average decrease of 18.7%. PD results appeared to be drug related in that the greatest improvements occurred approximately 24 hours after DM199 administration and subsequently declined.
In December 2019, we began enrolling patients in a Phase II CKD trial named REDUX, Latin for restore, a multi-center, open-label investigation of approximately 60 participants with CKD, who are being enrolled in two cohorts (30 per cohort). The study is being conducted in the United States at up to 10 sites and will be focused on participants with two specific causes of CKD. Cohort I of the study is focused on non-diabetic, hypertensive African Americans with Stage II or III CKD. African Americans are at greater risk for CKD than Caucasians, and those who have the APOL1 gene mutation are at an even higher risk. The study is designed to capture the APOL1 gene mutation as an exploratory biomarker in this cohort. Cohort II of the study is focused on participants with IgA Nephropathy (IgAN). The study will evaluate two dose levels of DM199 within each cohort. Study participants will receive DM199 by subcutaneous injection twice weekly for 95 days. The primary study endpoints include safety, tolerability, blood pressure, albuminuria and kidney function, which will be evaluated by changes from baseline in eGFR and albuminuria, as measured by the UACR.
In October 2019, we completed enrollment in the REMEDY trial, the Company’s Phase II study assessing the safety, tolerability and markers of therapeutic efficacy of DM199 in participants suffering from AIS. Final enrollment was 92 participants. The study was designed to measure safety and tolerability along with multiple tests designed to investigate DM199’s therapeutic potential including plasma-based biomarkers and standard functional stroke measures assessed at 90 days post-stroke. Standard functional stroke measurements include the Modified Rankin Scale, National Institutes of Health Stroke Scale, the Barthel Index and C-reactive protein, a measure of inflammation. These measurements are assessed at multiple points throughout the study, including 90 days post-stroke.
Potential DM199 Commercial Advantages
The growing understanding of KLK1’s role in human health and its use in Asia as an approved therapeutic highlights two important potential commercial advantages for DM199:
|
● |
KLK1 treatments currently sold in Japan, China and Korea. Research has shown that patients with low levels of KLK1 are associated with a variety of diseases related to vascular dysfunction, such as chronic kidney disease, acute ischemic stroke, retinopathy and hypertension. Clinical trial data with human urine and porcine derived KLK1 has demonstrated statistically significant clinical benefits in treating a variety of patients with KLK1 compared to placebo. These efficacy results are further substantiated by established markets in Japan, China and Korea for pharmaceutical sales of KLK1 derived from human urine and porcine pancreas. We estimate that millions of patients have been treated with these forms of KLK1 in Asia. Altogether, we believe this supports a strong market opportunity for a synthetic version of KLK1 such as DM199. |
|
● |
KLK1 treatment has had limited side effects and has been well tolerated to date. KLK1 is naturally produced by the human body; and, therefore, the body’s own control mechanisms act to limit potential side effects. The only notable side effect observed in our clinical trials was orthostatic hypotension, or a sudden drop in blood pressure, which was only seen at doses ten to twenty times higher than our anticipated therapeutic dose levels. Moreover, routine clinical use of KLK1 treatment in Asia we understand has been well-tolerated by patients for several decades. In 2017, we completed a clinical trial comparing the pharmacokinetic profile of DM199 to the human urinary form of KLK1 (Kailikang), which showed DM199, when administered in intravenous form, had a similar pharmacokinetic profile. Further, when DM199 was administered subcutaneously, DM199 demonstrated a longer acting pharmacokinetic profile, superior to the intravenously administered Kailikang and DM199. |
In addition, we believe there are also significant formulation, manufacturing, regulatory and other advantages for our synthetic human KLK1 drug candidate DM199:
|
● |
Potency and Impurity Considerations. KLK1 derived from human urine or porcine pancreas may contain impurities, endotoxins, and chemical byproducts due to the inherent variability of the isolation and purification process. We believe that this creates the risk of inconsistencies in potency and impurities from one production run to the next. However, we expect to produce a consistent formulation of KLK1 that is free of endotoxins and other impurities. |
|
● |
Cost and Scalability. Large quantities of human urine and porcine pancreas must be obtained to derive a small amount of KLK1. This creates potential procurement, cost and logistical challenges to source the necessary raw material, particularly for human urine sourced KLK1. Once sourced, the raw material is processed using chemicals and costly capital equipment and produces a significant amount of byproduct waste. Our novel recombinant manufacturing process utilizes widely available raw materials and can be readily scaled for commercial production. Accordingly, we believe our manufacturing process will have significant cost and scalability advantages. |
|
● |
Regulatory. We are not aware of any attempts by manufacturers of the urine or porcine based KLK1 products to pursue regulatory approvals in the United States. We believe that this is related to challenges presented by using inconsistent and potentially hazardous biomaterials, such as human urine and porcine pancreas, and their resulting ability to produce a consistent drug product. Our novel recombinant manufacturing process utilizes widely available raw materials which we believe provides a significant regulatory advantage, particularly in regions such as the United States, Europe and Canada, where safety standards are high. In addition, we believe that DM199 could qualify for 12 years of data exclusivity under the Biologics Price Competition and Innovation Act of 2009, which was enacted as part of the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act of 2010. |
Our Strategy
We aim to become a leader in the discovery, development and commercialization of recombinant proteins for the treatment of severe and life-threatening diseases. To achieve this goal, we are pursuing the following strategies:
|
● |
Complete our ongoing Phase II studies for DM199 in CKD patients; |
|
● |
Complete our ongoing Phase II study for DM199 in AIS patients; |
|
● |
Explore potential new indications for DM199; and |
|
● |
Leverage our experience and technologies to develop new recombinant therapies and programs. |
Our Team
We have assembled a seasoned management team with extensive experience in drug discovery, development and manufacturing. Our Chief Executive Officer, Rick Pauls, MBA, is a successful venture capitalist and formerly the Co-Founder and Managing Director of CentreStone Ventures Inc., a life sciences venture capital fund which made early investments in DiaMedica. Our Chief Medical Officer, Harry Alcorn Jr., PhD, has more than 30 years’ experience planning, operating, and executing clinical development programs across a range of diseases including kidney disease, diabetes, and cardiovascular disease, and most recently served as Chief Scientific Officer of DaVita Clinical Research. Our Vice President, Regulatory Affairs, Sydney Gilman, Ph.D., has more than 30 years’ experience in drug research, regulatory affairs and quality assurance, including six years as a chemistry reviewer in FDA’s Center for Drug Evaluation and Research. Edward Calamai, our consulting head of manufacturing, has over 30 years’ experience guiding manufacturing operations, including senior positions at Sensu and Seragen. Dr. Calamai is currently the Managing Partner at PM&C Associates, a company he co-founded in 2001. Our Chief Financial Officer, Scott Kellen, CPA, brings over two decades of operational and corporate finance expertise including an extensive background working with publicly-traded healthcare and biotechnology companies.
Risks Affecting Us
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus supplement. Some of these risks include:
|
● |
we are an early stage company with no approved products and no revenue from product sales; |
|
● |
our prospects depend on the success of our DM199 product candidate; |
|
● |
we rely on third parties to plan, conduct and monitor our preclinical and clinical trials; |
|
● |
we rely on a contract manufacturer over whom we have limited control to manufacture DM199; |
|
● |
clinical trials are expensive and complex with uncertain outcomes, which may prevent or delay regulatory approval for or commercialization of our DM199 product candidate; |
|
● |
we may not be successful in finding collaboration partners to assist us with the development or commercialization of our DM199 product candidate; |
|
● |
regulatory approval processes are lengthy, expensive and inherently unpredictable, and even if our DM199 product candidate achieves positive clinical trial results, we may fail to obtain required regulatory approvals; |
|
● |
we are in litigation with a contract research organization which could harm our ability to obtain regulatory approval for DM199; |
|
● |
even if we obtain required regulatory approvals, the successful commercialization of our DM199 product candidate may fail to achieve market acceptance among physicians, patients, healthcare payors and the medical community; |
|
● |
if we fail to obtain coverage and adequate reimbursement for our DM199 product candidate, our revenue-generating ability will be diminished and there is no assurance that the anticipated market for our product will be sustained; |
|
● |
we face competition from other biotechnology and pharmaceutical companies and may face such competition sooner than expected if we do not qualify for data exclusivity as anticipated; and |
|
● |
we may be classified as a “passive foreign investment company,” which may have adverse U.S. federal income tax consequences for U.S. shareholders. |
Recent Developments
We are in the process of finalizing our results for the year ended and as of December 31, 2019. These preliminary results represent our estimates which are based only on currently available information and do not present all necessary information for an understanding of our financial condition as of December 31, 2019 or our results of operations for the year ended December 31, 2019. This financial information has been prepared by and is the responsibility of our management. Our independent registered public accounting firm has not audited, reviewed or performed any procedures with respect to this preliminary financial data or the accounting treatment thereof and does not express an opinion or any other form of assurance with respect thereto. We expect to complete our audited financial statements for the year ended December 31, 2019 subsequent to the completion of this offering. While we are currently unaware of any items that would require us to make adjustments to the financial information set forth below, it is possible that we or our independent registered public accounting firm may identify such items as we complete our audited financial statements, and any resulting changes could be material. Accordingly, undue reliance should not be placed on these preliminary estimates. These preliminary estimates are not necessarily indicative of any future period and should be read together with “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements,” and our consolidated financial statements and related notes incorporated by reference in this prospectus supplement and the accompanying prospectus.
|
(in thousands) |
Year Ended December 31, 2019 |
|||
|
Research and development expense |
$ | 7,873 | ||
|
December 31, 2019 |
||||
|
Cash and cash equivalents |
3,883 | |||
|
Marketable securities |
3,995 | |||
|
Total cash and cash equivalents and marketable securities |
7,878 | |||
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion of revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act), and we may remain an emerging growth company for up to five years from December 31, 2018. However, if certain events occur prior to the end of such five-year period, including if we become a large accelerated filer, our annual gross revenue exceeds $1.07 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. In particular, we are required to only provide only two years of audited financial statements and are not required to disclose all of the executive compensation related information that would be required if we were not an emerging growth company. Accordingly, the information contained in our SEC reports may be different than the information you receive from other public companies in which you hold equity interests. However, we have irrevocably elected not to avail ourselves of the extended transition period for complying with new or revised accounting standards, and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Company Information
Our principal executive offices are located at Two Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447. Our telephone number is (763) 312-6755, and our Internet website address is www.diamedica.com. We make available on our website free of charge a link to our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as practicable after we electronically file such material with the SEC. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We are a corporation governed under the British Columbia Business Corporations Act (BCBCA). Our company was initially incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of incorporation dated January 21, 2000. Our articles were amended (i) on February 26, 2001 to change our corporate name to DiaMedica Inc., (ii) on April 11, 2016 to continue the Company from The Corporations Act (Manitoba) to the Canada Business Corporations Act (CBCA), (iii) on December 28, 2016 to change our corporate name to DiaMedica Therapeutics Inc., (iv) on September 24, 2018 to permit us to hold shareholder meetings in the U.S. and to permit our directors, between annual general meetings of our shareholders, to appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of shareholders, (v) on November 15, 2018 to effect a 1-for-20 consolidation of our common shares, and (vi) on May 31, 2019, to continue our existence from a corporation incorporated under the CBCA into British Columbia under the BCBCA.
The Offering
The following summary contains general information about this offering. The summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus supplement and the accompanying prospectus.
|
Common shares offered by us |
2,125,000 shares. |
|
Offering price |
$4.00 per common share. |
|
Common shares to be outstanding immediately after this offering |
14,131,874 shares. |
|
Use of proceeds |
We estimate that our net proceeds from this offering will be approximately $7.7 million after deducting the underwriting discount and the estimated offering expenses payable by us. We intend to use the net proceeds from this offering to continue our clinical and product development activities and for other working capital and general corporate purposes. See “Use of Proceeds.” |
|
Risk factors |
Investing in our common shares involves a high degree of risk. See “Risk Factors” and other information incorporated by reference in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully consider before deciding to invest in our common shares. |
|
Nasdaq Capital Market symbol |
“DMAC” |
The number of our common shares to be outstanding immediately after this offering, as shown above, is based on 12,006,874 common shares issued and outstanding as of September 30, 2019, and excludes as of that date the following:
|
● |
1,012,563 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.44 per share; |
|
● |
624,568 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.10 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
627,325 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,372,675 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below before making a decision about investing in our securities. The risks and uncertainties discussed below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business. If any of these risks occur, our business, financial condition and operating results could be harmed, the trading price of our common shares could decline, and you could lose part or all of your investment.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred substantial losses since our inception and expect to continue to incur future substantial losses and may never become profitable.
We are a clinical stage biopharmaceutical company focused on the development of novel recombinant proteins. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate will fail to prove effective, gain regulatory approval or become commercially viable. We do not have any products approved by regulatory authorities and have not generated any revenues from product sales to date, and do not expect to generate any revenue from the sale of products for several years. We have incurred significant research, development and other expenses related to our ongoing operations and expect to continue to incur such expenses. As a result, we have not been profitable and have incurred significant operating losses in every reporting period since our inception. For the year ended December 31, 2018 and 2017, we incurred a net loss of $5.7 million and $4.3 million, respectively. As of September 30, 2019, we had an accumulated deficit of $54.1 million. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our shareholders’ equity and working capital. We expect to continue to incur substantial operating losses as we continue our research and development (R&D) activities, planned clinical trials, regulatory activities and otherwise develop our product candidate, DM199, or any future product candidates to a point where they may be commercially sold and we begin to recognize future product sales, or receive royalty payments, licensing fees, and/or milestone payments sufficient to generate revenues to fund our continuing operations. We expect our operating losses to increase in the near term as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. We are unable to predict the extent of any future losses or when we will become profitable, if ever. Our failure to become and remain profitable may depress the market price of our common shares and could impair our ability to raise capital, develop products, expand our business and product offerings or continue our operations. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We currently have no revenue from product sales and do not expect any revenue from product sales for several years. Accordingly, we will need additional funding to continue our research and development activities and other operations, which may not be available to us on acceptable terms, or at all.
Our future operations will be dependent upon our ability to develop our product candidates, obtain research grant funding, obtain required regulatory approvals, generate revenue from product sales, negotiate collaboration or license agreements or other strategic alternatives, and/or secure additional funding. As of September 30, 2019, we had cash, cash equivalents and marketable securities of $9.7 million. We expect we will need substantial additional capital to further our R&D activities, planned clinical trials, and regulatory activities and to otherwise develop our product candidate, DM199, or any future product candidates to a point where they may be commercially sold. While we are striving to achieve these plans, there is no assurance we will be successful or that additional financing will be obtained on favorable terms or at all in furtherance of our strategic objectives. Our ability to continue as a going concern is dependent on our ability to continue obtaining sufficient funds to conduct our R&D activities and to successfully commercialize our product candidates.
We will require additional funds to finance our operations, which may not be available to us on acceptable terms, or at all. As a result, we may not complete the development and commercialization of our current DM199 product candidate or develop any new product candidates.
We require significant additional funds for further R&D activities, planned clinical trials and the regulatory approval process. We expect our cash resources of $9.7 million in cash, cash equivalents and marketable securities as of September 30, 2019 to be sufficient to allow us to complete our current ongoing Phase II REMEDY trial in patients with AIS and the first two cohorts in the Phase II study in patients with CKD and to otherwise fund our planned operations into the fourth quarter of 2020. However, the amount and timing of future funding requirements will depend on many factors, including, among others:
|
● |
the rate of progress in the development of and the conduct of clinical trials with respect to our DM199 product candidate and any other future product candidates; |
|
● |
the timing and results of our ongoing development efforts, including in particular our current Phase II clinical studies; |
|
● |
the costs of our development efforts, including the conduct of clinical trials with respect to our DM199 product candidate and any other future product candidates; |
|
● |
the costs associated with identifying additional product candidates and the potential expansion of our current development programs or potential new development programs; |
|
● |
the costs to initiate and continue research, preclinical, and clinical development efforts for any future product candidates; |
|
● |
the costs necessary to obtain regulatory approvals for our DM199 product candidate and any other future product candidates; |
|
● |
the costs associated with being a public company; |
|
● |
the costs we incur in the filing, prosecution, maintenance and defense of our intellectual property; and |
|
● |
the costs related to general and administrative (G&A) support. |
We may require significant additional funds earlier than we currently expect, and there is no assurance that we will not need or seek additional funding prior to such time. We may elect to raise additional funds even before we need them if market conditions for raising additional capital are favorable.
Since our inception, we have financed our operations from public and private sales of equity securities, the exercise of warrants and stock options, interest income on funds available for investment, and government grants and tax incentives, and we expect to continue this practice for the foreseeable future. We do not have any existing credit facilities under which we could borrow funds. We may seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This is particularly true if our clinical data is not positive or economic and market conditions deteriorate.
Although we have previously been successful in obtaining financing through our equity securities offerings, there can be no assurance that we will be able to do so in the future. To the extent we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. Debt financing, if available, may involve agreements that include conversion discounts or covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, or strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. It is possible that financing will not be available or, if available, may not be on favorable terms. The availability of financing will be affected by the results of our clinical studies and other scientific and clinical research; our ability to attain regulatory approvals; market acceptance of our product candidates; the state of the capital markets generally with particular reference to pharmaceutical, biotechnology, and medical companies; the status of strategic alliance agreements; and other relevant commercial considerations. If adequate funding is not available, we may be required to implement cost reduction strategies; delay, reduce, or eliminate one or more of our product development programs; relinquish significant rights to product candidates or obtain funds on less favorable terms than we would otherwise accept; and/or divest assets or cease operations through a merger, sale, or liquidation of our company.
We are exposed to financial risk related to the fluctuation of foreign currency exchange rates and the degrees of volatility of those rates.
We may be adversely affected by foreign currency exchange rate fluctuations. To date, we have been primarily funded through issuances of equity and proceeds from the exercise of warrants and stock options, which are denominated both in U.S. and Canadian dollars. Currently, the majority of our expenditures are in U.S. dollars. However, significant costs are also incurred in Canadian dollars, British pounds, and Australian dollars, and, therefore, we are subject to foreign currency exchange rate fluctuations which may, from time to time, impact our financial position and results of operations.
Risks Related to Our Business and Our Industry
We are an early stage company with no approved products and no revenue from commercialization of any products.
We are at an early stage of development of our product candidate, DM199, for the treatment of CKD and AIS. We have not completed the development of any product candidate and, accordingly, have not begun to commercialize any product candidate or generate any revenues from any product sales. DM199 requires significant additional clinical testing and investment prior to seeking marketing approval. A commitment of substantial resources by us and potential partners to continue to conduct clinical trials for DM199 will be required to meet applicable regulatory standards, obtain required regulatory approvals, and successfully commercialize this product candidate. DM199 is not expected to be commercially available for several years, if at all.
Our prospects depend on the success of our product candidate, DM199, which is at an early stage of development, and we may not generate revenue from product sales for several years, if at all, from this product candidate or any future product candidates.
We are highly dependent on the success of DM199, and we may not be able to successfully obtain regulatory or marketing approval for, or successfully commercialize, this product candidate. To date, we have expended significant time, resources and effort on the development of DM199, including conducting preclinical and clinical trials, for the treatment of CKD and AIS. Although we intend to study the use of DM199 to treat multiple diseases, we have no other product candidates in our current clinical development pipeline. Our ability to generate revenue from product sales and to achieve commercial success in the near term will initially depend almost entirely on our ability to successfully develop, obtain regulatory approval for and then successfully commercialize DM199. Prior to commercialization of any potential product, significant additional investments will be necessary to complete the development of DM199 or any future product candidates. Preclinical and clinical trial work must be completed before DM199 or any future product candidate could be ready for use within the markets that we have identified. We may fail to develop any products, obtain regulatory approvals, complete required clinical trials successfully, or commercialize any products. Competitors may develop alternative products and methodologies to diagnose and treat the disease indications we are pursuing, thus reducing our competitive advantages. We do not know whether any of our product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost, or be successfully marketed. The product candidate we are currently developing is not expected to be commercially viable for several years. In addition, although no significant adverse events have occurred to date, DM199 may cause undesirable side effects. Results of early preclinical and clinical research may not be indicative of the results that will be obtained in later stages of clinical research. If regulatory authorities do not approve DM199 for the treatment of CKD and/or AIS or any future product candidates, or if we fail to maintain regulatory compliance, we will have limited ability to commercialize DM199 or any future product candidates, and our business and results of operations would be harmed. If we do succeed in developing viable products from DM199 or any future product candidates, we will face many potential obstacles, such as the need to develop or obtain manufacturing, sales and marketing, and distribution capabilities.
The clinical and commercial success of our DM199 product candidate will depend on a number of factors, many of which are beyond our control.
The clinical and commercial success of our DM199 product candidate will depend on a number of factors, many of which are beyond our control, including, among others:
|
● |
the timely initiation, continuation, and completion of our currently ongoing Phase II and future clinical trials for DM199, which will depend substantially upon requirements for such trials imposed by the FDA and other regulatory agencies and bodies; |
|
● |
our ability to demonstrate the safety and efficacy of DM199 to the satisfaction of the relevant regulatory authorities; |
|
● |
whether we are required by the FDA or other regulatory authorities to conduct additional clinical trials, and the scope and nature of such clinical trials, prior to approval to market our DM199 product candidate; |
|
● |
the timely receipt of necessary marketing approvals from the FDA and foreign regulatory authorities, including pricing and reimbursement determinations; |
|
● |
the ability to successfully commercialize our DM199 product candidate for marketing and sale, if approved by the FDA or foreign regulatory authorities, whether alone or in collaboration with others; |
|
● |
our ability and the ability of third-party manufacturers to manufacture the quantities of our DM199 product candidate with quality attributes necessary to meet regulatory requirements and at a scale and yield sufficient to meet anticipated demand at a cost that allows us to achieve profitability; |
|
● |
our success in educating health care providers and patients about the benefits, risks, administration, and use of our DM199 product candidate, if approved; |
|
● |
acceptance of our DM199 product candidate, if approved, as safe and effective by patients and the healthcare community; |
|
● |
the achievement and maintenance of compliance with all regulatory requirements applicable to our DM199 product candidate, our third-party manufacturers, and our internal operations; |
|
● |
the maintenance of an acceptable safety profile of our products, if any, following any approval; |
|
● |
the availability, perceived advantages, relative cost, relative safety, and relative efficacy of alternative and competitive treatments; |
|
● |
our ability to provide approved product with a convenient and patient-friendly administration procedure; |
|
● |
our ability to successfully enforce our intellectual property rights for our DM199 product candidate and against the products of potential competitors; and |
|
● |
our ability to avoid or succeed in third-party patent interference or patent infringement claims. |
We cannot assure you that we will ever be able to achieve profitability through the sale of, or royalties from, our DM199 product candidate. If we or our future collaborators are not successful in obtaining approval for and commercializing our DM199 product candidate, or are delayed in completing those efforts, our business and operations would be adversely affected.
We rely and will continue to rely on third parties to plan, conduct, and monitor our preclinical and clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies in specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring, and project management. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs may face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, cancelled, or rendered ineffective.
We rely on a contract manufacturer over whom we have limited control. If we are subject to quality, cost, or delivery issues with the materials supplied by this or future contract manufacturers, our business operations could suffer significant harm.
Completion of our clinical trials and commercialization of our DM199 product candidate and any future product candidates require access to, or development of, facilities to manufacture our product candidates at sufficient yields and at commercial scale. Our clinical trials must be conducted with product candidates produced under applicable current good manufacturing practices (cGMP) regulations. Failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. We rely on contract manufacturing organizations (CMOs) to manufacture DM199. We rely on CMOs for manufacturing, filling, packaging, storing, and shipping DM199 in compliance cGMP regulations applicable to DM199. The FDA ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packing of a drug product.
We have no direct experience in manufacturing or managing third parties in manufacturing our DM199 product candidate in the volumes that are expected to be necessary to support our clinical trials and commercialization, if DM199 is approved. Our efforts to establish these capabilities may not meet our requirements as to scale-up, timeliness, yield, cost, or quality in compliance with cGMP regulations applicable to DM199. We, our future collaborators, or our experienced third-party manufacturers may encounter difficulties in production, which may include the following, among others:
|
● |
costs and challenges associated with scale-up and attaining sufficient manufacturing yields; |
|
● |
supply chain issues, including the timely availability and shelf life requirements of raw materials and supplies and the lack of redundant and backup suppliers; |
|
● |
quality control and assurance; |
|
● |
shortages of qualified personnel and capital required to manufacture large quantities of our product candidate; |
|
● |
competing capacity needs at CMOs supporting product development as quantities for supply increase; |
|
● |
establishment of commercial supply capacity through binding supply agreements; |
|
● |
compliance with regulatory requirements that vary in each country where a product might be sold; |
|
● |
capacity limitations and scheduling availability in contracted facilities; and |
|
● |
natural disasters, cyberattacks, or other force majeure events that affect facilities and possibly limit production or loss of product inventory maintained in third party storage facilities. |
There can be no assurances that our current CMOs or any future CMOs will be able to meet our timetable and requirements for our DM199 product candidate or any future product candidates. If we are unable to arrange for alternative third-party manufacturing sources on commercially reasonable terms or in a timely manner, we may be delayed in the development of DM199 and any future product candidates. Further, CMOs must operate in compliance with cGMP regulations, and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon our current CMOs and any future third parties for the manufacture of our product candidates may adversely affect our ability to develop our product candidates on a timely and competitive basis and, if we are able to commercialize our product candidates, may adversely affect our revenues from product sales and profit margins.
If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we would incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct preclinical studies and extensive clinical trials in humans to demonstrate the safety and efficacy of our product candidates. Clinical testing is expensive and difficult to design and implement, can take many years to complete, and has uncertain outcomes. The outcome of preclinical studies and early clinical trials may not predict the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety profiles, notwithstanding promising results in earlier trials. We do not know whether the clinical trials we are currently conducting or may conduct in the future will demonstrate adequate efficacy and safety to result in regulatory approval to market DM199 or any future product candidates in any jurisdiction. A product candidate may fail for safety or efficacy reasons at any stage of the testing process. In addition, the patient populations in our clinical studies for DM199 often have many co-morbidities that may cause severe illness or death, which may be attributed to DM199 in a manner that negatively affects the safety profile of our DM199 product candidate. If the results of our ongoing or future clinical trials for DM199 are inconclusive with respect to efficacy, if we do not meet our clinical endpoints with statistical significance, or if there are unanticipated safety concerns or adverse events that emerge during clinical trials, we may be prevented from or delayed in obtaining marketing approval, and even if we obtain marketing approval, any sales may suffer. A major risk we face is the possibility that neither our current DM199 product candidate or any future product candidates will successfully gain market approval from the FDA or other regulatory authorities, resulting in us being unable to derive any commercial revenue from them after investing significant amounts of capital in multiple stages of preclinical and clinical testing.
If we experience delays in clinical testing, we will be delayed in commercializing our product candidates, and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, or at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The commencement and completion of clinical trials for our product candidates may be delayed for a number of reasons, including among others:
|
● |
failure by regulatory authorities to grant permission to proceed or placing the clinical trial on hold; |
|
● |
patients failing to enroll or remain in our trials at the rates and within the timelines we expect; |
|
● |
suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with cGMP requirements; |
|
● |
any changes to our manufacturing process that may be necessary or desired; |
|
● |
delays or failure to obtain clinical supply from contract manufacturers of our product candidates necessary to conduct clinical trials; |
|
● |
product candidates demonstrating a lack of safety or efficacy during clinical trials; |
|
● |
patients choosing an alternative treatment for the indications for which we are developing any of our product candidates or participating in competing clinical trials; |
|
● |
patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects, or other reasons; |
|
● |
reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
|
● |
competing clinical trials and scheduling conflicts with participating clinicians; |
|
● |
clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
|
● |
failure of our contract research organizations (CROs) to satisfy their contractual duties or meet expected deadlines; |
|
● |
inspections of clinical trial sites by regulatory authorities, Institutional Review Boards (IRBs) or ethics committees finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
|
● |
one or more IRBs or ethics committees rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
|
● |
failure to reach agreement on acceptable terms with prospective clinical trial sites. |
We are currently experiencing slower than expected enrollment in our pending Phase II studies for CKD. We believe that this was due to the Thanksgiving and Christmas holidays. However, if this slow enrollment continues, the completion of these studies will take longer than expected.
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees for re-examination, which may impact the cost, timing or successful completion of that trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition, and prospects.
Even if we complete the necessary preclinical studies and clinical trials, the regulatory approval process is expensive, time-consuming and uncertain and may prevent us or any future collaborators from obtaining approvals for the commercialization of some or all of our product candidates. As a result, we cannot predict when or if, and in which territories, we, or any future collaborators, will obtain marketing approval to commercialize a product candidate.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive and may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved.
Our current product candidate and the activities associated with its development and commercialization, including design, research, testing, manufacture, safety, efficacy, quality control, recordkeeping, labeling, packaging, storage, approval, advertising, promotion, sale, distribution, import, export, and reporting of safety and other post-market information, are subject to comprehensive regulation by the FDA, the European Medicines Agency (EMA) and other similar foreign regulatory agencies. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-parties to assist us in this process. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. The FDA, EMA or other regulatory authorities may determine that our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use. As a result, any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
In addition, changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate.
We are in litigation against Pharmaceutical Research Associates Group B.V., a contract research organization, seeking to compel them to comply with the terms of a clinical trial research agreement, and their failure to perform as required could adversely affect our ability to obtain regulatory approval for DM199.
In March 2013, we entered into a clinical research agreement with Pharmaceutical Research Associates Group B.V. (PRA Netherlands) to perform a double-blinded, placebo-controlled, single-dose and multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and proof of concept of DM199 in healthy subjects and in patients with Type 2 diabetes mellitus. In one arm of this study, we enrolled 36 patients with Type 2 diabetes who were treated with two subcutaneous dose levels of DM199 over a 28-day period. This study achieved its primary endpoint and demonstrated that DM199 was well tolerated. The secondary endpoints for this study, however, were not met. We believe there were significant execution errors in Part D of the study that were caused by protocol deviations occurring at the clinical trial site that were unable to be reconciled. We believe these included dosing errors and sample mix-ups. These errors undermined our ability to interpret the secondary endpoints. To date, we have been unable to obtain the complete study records from PRA Netherlands for the arm of the study that included 36 patients with Type 2 diabetes and was intended to measure primary endpoints (safety and tolerability) and secondary endpoints (blood glucose concentration, insulin levels, glucose tolerance test and a variety of experimental biomarkers). Without these records and given our inability to reconcile the protocol deviations, we have been unable to generate a final study report. Due in part to these confounded secondary endpoints, we are not currently continuing the clinical study of DM199 for Type 2 diabetes. We have initiated litigation against PRA Netherlands to compel them to comply with the terms of the clinical research agreement, including providing full study records, and to recover damages. Litigation distracts the attention of our management from our business, is expensive and the outcome is uncertain.
Though we have entered into a litigation funding agreement to help cover the costs associated with our litigation against PRA Netherlands, there is no assurance that we will generate any recovery from the litigation and, because of the terms of the litigation funding agreement, a significant portion, if not all, of any recovery we may obtain may be due to the funder under the agreement.
On December 27, 2019, we entered into a litigation funding agreement with LEGALIST FUND II, L.P. (Funder) for the purpose of funding our currently pending lawsuit against PRA Netherlands. Our management believes, but cannot guarantee, that this litigation funding agreement will allow us to pursue this litigation more effectively. Although the Funder made its evaluation as to the likelihood of success, litigation is very uncertain, and we cannot assure you that, just because we have obtained litigation funding, we will be successful or that any recovery we may obtain will be significant.
Under the terms of the litigation funding agreement, the Funder agreed to pay up to an aggregate of $1.0 million to fund reasonable legal fees, court costs, and other expenses incurred by us in connection with the litigation, including $200,000 for fees and costs previously paid by us. These payments, however, are conditioned upon the transfer of venue of the litigation from Delaware to Minnesota (Transfer), and if the venue is not transferred, we will not be entitled to receive any payments under the litigation funding agreement. If the venue is transferred, we agreed to repay the Funder from any proceeds arising from the litigation (Claim Proceeds) the amount of costs actually paid or otherwise funded by the Funder in connection with the litigation, plus the reimbursement of $10,000 for its diligence and underwriting costs. Additionally, we agreed to pay the Funder from the Claim Proceeds the greater of: (i) $1.0 million if repayment occurs within nine months of the Transfer, $2.0 million if repayment occurs more than nine months after the Transfer but before trial has begun, or $3.0 million thereafter; or (ii) 20% of the Claim Proceeds. In the event the Funder has not been repaid 3½ years after the Transfer, the Funder is entitled to receive interest on the unpaid amounts equal to 20% per annum commencing on the 3½ year anniversary of the Transfer. Our obligation under the litigation funding agreement to make the foregoing payments to the Funder is non-recourse and limited only to the Claim Proceeds. As a result of the agreement, if we obtain Claim Proceeds, it is possible, depending on the amount of the Claim Proceeds, that we will receive no net recovery after all payments have been made to the Funder.
We may not be able to obtain FDA acceptance of INDs to commence future clinical trials in the United States on the timelines we expect, and even if we are able to, the FDA may not permit us to proceed in a timely manner, or at all.
Prior to commencing clinical trials in the United States for future trials of our current DM199 product candidate or any trials of future product candidates, we will be required to have an accepted IND for each product candidate and for each targeted indication. During 2019, we filed, and the FDA accepted, an IND for the Phase Ib study and the first two cohorts in the Phase II study in patients with CKD. A submission of an IND may not result in the FDA allowing further clinical trials to begin and, once begun, issues may arise that will require us to suspend or terminate such clinical trials. Additionally, even if relevant regulatory authorities agree with the design and implementation of the clinical trials set forth in an IND, these regulatory authorities may change their requirements in the future. Failure to submit or obtain acceptance of INDs may cause the development of our product candidates to be delayed or terminated, which could materially and adversely affect our business and prospects.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or not completed at all.
As DM199 and any future product candidates advance to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials on a timely basis or at all. The factors that affect our ability to enroll patients are largely uncontrollable and include, among others:
|
● |
size and nature of the patient population; |
|
● |
eligibility and exclusion criteria for the trial; |
|
● |
design of the study protocol; |
|
● |
competition with other companies for clinical sites or patients; |
|
● |
the perceived risks and benefits of the product candidate under study; |
|
● |
real or perceived availability of alternative treatments; |
|
● |
the patient referral practices of physicians; |
|
● |
the number, availability, location, and accessibility of clinical trial sites; and |
|
● |
the efforts of our physician investigators and clinical trial sites to facilitate enrollment in our clinical trials. |
We are currently experiencing slower than expected enrollment in our pending Phase II studies for CKD. We believe that this was due to the Thanksgiving and Christmas holidays. However, if this slow enrollment continues, the completion of these studies will take longer than expected. We may not be able to successfully initiate or continue clinical trials if we cannot timely enroll a sufficient number of eligible patients to participate in the clinical trials required by regulatory agencies. If we have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay, limit, or terminate on-going or planned clinical trials, any of which could have a material adverse effect on our business and prospects and ability to raise additional financing to fund our operations.
We may not be able to reproduce the results of previously conducted clinical studies of other forms of KLK1, including Kailikang and Kallidinogenase, thereby preventing DM199 from displacing other forms of KLK1.
While there have been numerous studies demonstrating the efficacy of Kailikang and Kallidinogenase, we rely on the scientific and clinical knowledge and experience of other biotechnology and pharmaceutical companies and organizations in conducting those clinical studies. No assurance can be given that in our clinical trials involving DM199 we will be able to reproduce results of previously conducted studies or prove that DM199 is safe or effective and able to displace other forms of KLK1 in the market.
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our product candidates may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors, or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect the market price of our common shares and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive, or the trials are not well designed.
Clinical trials must be conducted in accordance with the FDA’s current Good Clinical Practices requirements, or cGCPs, or analogous requirements of applicable foreign regulatory authorities. Clinical trials are subject to oversight by the FDA, other foreign governmental agencies, and IRBs or ethics committees at the study sites where the clinical trials are conducted. In addition, clinical trials must be conducted with product candidates produced in accordance with applicable current Good Manufacturing Practices. Clinical trials may be suspended by us or by the FDA, other foreign regulatory authorities, or by an IRB or ethic committee with respect to a particular clinical trial site, for various reasons, including:
|
● |
deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with regulatory requirements or study protocols; |
|
● |
deficiencies in the clinical trial operations or trial sites; |
|
● |
unforeseen adverse side effects or the emergence of undue risks to study subjects; |
|
● |
deficiencies in the trial design necessary to demonstrate efficacy; |
|
● |
the product candidate may not appear to offer benefits over current therapies; or |
|
● |
the quality or stability of the product candidate may fall below acceptable standards. |
The design and implementation of clinical trials is a complex process. As a Company, we have limited experience designing and implementing clinical trials. We may not successfully or cost-effectively design and implement clinical trials that achieve our desired clinical endpoints efficiently, or at all. A clinical trial that is not well designed may delay or even prevent initiation of the trial, can lead to increased difficulty in enrolling patients, may make it more difficult to obtain regulatory approval for the product candidate on the basis of the study results, or, even if a product candidate is approved, could make it more difficult to commercialize the product successfully or obtain reimbursement from third party payers. Additionally, a trial that is not well-designed could be inefficient or more expensive than it otherwise would have been, or we may incorrectly estimate the costs to implement the clinical trial, which could lead to a shortfall in funding.
Regulatory approval processes are lengthy, expensive, and inherently unpredictable. Our inability to obtain regulatory approval for our product candidates would substantially harm our business.
Our shareholders and investors should be aware of the risks, problems, delays, expenses, and difficulties which we may encounter in light of the extensive regulatory environment within which our business is carried out. Numerous statutes and regulations govern the preclinical and clinical development, manufacture and sale, and post-marketing responsibilities for non-therapeutic and human therapeutic products in the United States, European Union and other countries that are the intended markets for our current and future product candidates. Such legislation and regulations govern the approval of manufacturing facilities, the testing procedures, and controlled research that must be carried out, and the preclinical and clinical data that must be collected prior to marketing approval. Our R&D efforts, as well as any future clinical trials, and the manufacturing and marketing of any products we may develop, will be subject to and restricted by such extensive regulations.
The process of obtaining necessary regulatory approvals is lengthy, expensive, and uncertain. We may fail to obtain the necessary approvals to commence or continue clinical testing or to manufacture or market our potential products in reasonable time frames, if at all. In addition, governmental authorities in the United States or other countries may enact regulatory reforms or restrictions on the development of new therapies that could adversely affect the regulatory environment in which we operate or the development of any products we may develop.
Completing clinical testing and obtaining required approvals is expected to take several years and to require the expenditure of substantial resources. There can be no assurance that clinical trials will be completed successfully within any specified period of time, if at all. Furthermore, clinical trials may be delayed or suspended at any time by us or by the various regulatory authorities if it is determined at any time that the subjects or patients are being exposed to unacceptable risks.
Any failure or delay in obtaining regulatory approvals would adversely affect our ability to utilize our technology and would adversely affect our operations. Furthermore, no assurance can be given that our current or future product candidates will prove to be safe and effective in clinical trials or that they will receive the requisite regulatory approval. Moreover, any regulatory approval of a drug that is eventually obtained may be granted with specific limitations on the indicated uses for which that drug may be marketed. Furthermore, product approvals may be withdrawn if problems occur following initial marketing or if compliance with regulatory standards is not maintained.
Any product candidate for which we obtain marketing approval could be subject to post-marketing restrictions or recall or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any of them are approved.
The FDA and other federal and state agencies, including the U.S. Department of Justice (DOJ), closely regulate compliance with all requirements governing prescription drug products, including requirements pertaining to marketing and promotion of drugs in accordance with the provisions of the approved labeling and manufacturing of products in accordance with cGMP requirements. The FDA and DOJ impose stringent restrictions on manufacturers’ communications regarding off-label use, and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing. Violations of such requirements may lead to investigations alleging violations of the Food, Drug and Cosmetic Act and other statutes, including the False Claims Act and other federal and state health care fraud and abuse laws as well as state consumer protection laws.
Our failure to comply with all regulatory requirements, and later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, may yield various results, including:
|
● |
litigation involving patients using our products; |
|
● |
restrictions on such products, manufacturers or manufacturing processes; |
|
● |
restrictions on the labeling or marketing of a product; |
|
● |
restrictions on product distribution or use; |
|
● |
requirements to conduct post-marketing studies or clinical trials; |
|
● |
warning or untitled letters; |
|
● |
withdrawal of the products from the market; |
|
● |
refusal to approve pending applications or supplements to approved applications that we submit; |
|
● |
recall of products; |
|
● |
fines, restitution or disgorgement of profits or revenues; |
|
● |
suspension or withdrawal of marketing approvals; |
|
● |
damage to relationships with any potential collaborators; |
|
● |
unfavorable press coverage and damage to our reputation; |
|
● |
refusal to permit the import or export of our products; |
|
● |
product seizure; or |
|
● |
injunctions or the imposition of civil or criminal penalties. |
Non-compliance by us or any future collaborator with regulatory requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with regulatory requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
We may not achieve our publicly announced milestones according to schedule, or at all.
From time to time, we may announce the timing of certain events we expect to occur, such as the anticipated timing of initiation or completion of or results from our clinical trials. These statements are forward-looking and are based on the best estimates of management at the time relating to the occurrence of such events. However, the actual timing of such events may differ significantly from what has been publicly disclosed. The timing of events such as the initiation or completion of a clinical trial, filing of an application to obtain regulatory approval, or an announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a CMO or CRO or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business plan, financial condition or operating results, and the trading price of our common shares.
Future development collaborations may be important to us. If we are unable to enter into or maintain these collaborations, or if these collaborations are not successful, our business could be adversely affected.
We may in the future determine to seek to collaborate with pharmaceutical and biotechnology companies for the development or commercialization of our current or future product candidates. We face significant competition in seeking appropriate collaborators. Our ability to reach a definitive agreement for any collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential development schedule or reduce the scope of research activities, or increase our expenditures and undertake discovery, nonclinical or clinical development activities at our own expense. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development activities, we may not be able to continue or further develop our current or future product candidates, and our business may be materially and adversely affected.
Future collaborations we may enter into may involve the following risks, among others:
|
● |
collaborators may have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
|
● |
collaborators may not perform their obligations as expected; |
|
● |
changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, may divert resources or create competing priorities; |
|
● |
collaborators may delay discovery, nonclinical or clinical development, provide insufficient funding for product development of targets selected by us, stop or abandon discovery, nonclinical or clinical development for a product candidate, or repeat or conduct new discovery, and nonclinical and clinical development for a product candidate; |
|
● |
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed than our products; |
|
● |
product candidates discovered in collaboration with us may be viewed by our future collaborators as competitive with their own product candidates or products, which may cause collaborators to cease to devote resources to the development of our product candidates; |
|
● |
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the discovery, preclinical or clinical development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time-consuming and expensive; |
|
● |
collaborators may not properly maintain or defend our intellectual property rights or intellectual property rights licensed to us or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
|
● |
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and |
|
● |
collaborations may be terminated for the convenience of the collaborator and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates. |
Additionally, subject to its contractual obligations to us, if a collaborator is involved in a business combination, the collaborator might deemphasize or terminate the development of any of our product candidates. If a collaborator terminates its agreement with us, we may find it more difficult to attract new collaborators and the way we are perceived in the business and financial communities could be adversely affected.
If our collaborations do not result in the successful development of our product candidates, our product candidates could be delayed and we may need additional resources to develop product candidates. All of the risks relating to product development, regulatory approval and commercialization described in this report also apply to the activities of our future collaborators.
The successful commercialization of our current or future product candidates, if approved, will depend on achieving market acceptance, and we may not be able to gain sufficient acceptance to generate significant revenue.
Even if our product candidates are successfully developed and receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payers, such as private insurers or governments and other funding parties, and the medical community. The degree of market acceptance for any products we develop will depend on a number of factors including, among others:
|
● |
demonstration of the clinical efficacy and safety; |
|
● |
the prevalence and severity of any adverse side effects; |
|
● |
limitations or warnings contained in the product’s approved labeling; |
|
● |
cost-effectiveness and availability of acceptable pricing; |
|
● |
competitive product profile versus alternative treatment methods and the superiority of alternative treatment or therapeutics; |
|
● |
the effectiveness of marketing and distribution methods and support for the products; and |
|
● |
coverage and reimbursement policies of government and third-party payers to the extent that our products could receive regulatory approval but not be approved for coverage by or receive adequate reimbursement from government and quasi-government agencies or other third-party payers. |
Disease indications may be small subsets of a disease that could be parsed into smaller and smaller indications as different subsets of diseases are defined. This increasingly fine characterization of diseases could have negative consequences, including creating an approved indication that is so small as not to have a viable market for us. If future technology allows characterization of a disease in a way that is different from the characterization used for large, pivotal studies, it may make those studies invalid or reduce their usefulness and may require repeating all or a portion of the studies. Future technology may supply better prognostic ability, which could reduce the portion of patients projected to need a new therapy. Even after being cleared by regulatory authorities, a product may later be shown to be unsafe or not to have its purported effect, thereby preventing its widespread use or requiring withdrawal from the market.
If we fail to obtain coverage and adequate reimbursement for our products, our revenue-generating ability will be diminished, and there is no assurance that the anticipated market for our products will be sustained.
We believe that there may be many different potential applications for products successfully derived from our technologies and that the anticipated market for products under development will continue to expand. However, due to competition from existing or new products and the yet-to-be established commercial viability of our product candidates, no assurance can be given that these beliefs will prove to be correct. Physicians, patients, formularies, payers or the medical community in general may not accept or utilize any products that we may develop. Other drugs may be approved during our clinical testing, which could change the accepted treatments for the diseases we have targeted and make our product candidates obsolete.
Our ability to successfully commercialize our future products, if any, will depend, in part, on the extent to which coverage of and adequate reimbursement for such products and related treatments will be available from governmental health payer programs at the federal and state levels, including Medicare and Medicaid, private health insurers, managed care plans and other organizations. No assurance can be given that third-party coverage and adequate reimbursement will be available that will allow us to obtain or maintain price levels sufficient for the realization of an appropriate return on our investment in product development. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and private health insurers, managed care plans and other organizations is critical to new product acceptance by healthcare providers. There is no uniform coverage and reimbursement policy among third-party payers in the United States; however, private third-party payers may follow Medicare coverage and reimbursement policy in setting their own coverage policy and reimbursement rates. Additionally, coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Even if we obtain coverage for our product candidates, the related reimbursement rates might not be adequate to make our product attractive to providers, or may require patient cost sharing (e.g., copayments/deductibles) that patients find unacceptably high. In addition, healthcare reform and controls on healthcare spending may limit coverage of our products and the price we charge and get paid for any products and the amounts thereof that we can sell. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates.
Outside of the United States, the successful commercialization of our products will depend largely on obtaining and maintaining government coverage, because in many countries patients are unlikely to use prescription drugs that are not covered by their government healthcare programs. Negotiating coverage and reimbursement with governmental authorities can delay commercialization by 12 months or more. Coverage and reimbursement policies may adversely affect our ability to sell our products on a profitable basis. In many international markets, governments control the prices of prescription pharmaceuticals, including through the implementation of reference pricing, price cuts, rebates, revenue-related taxes and profit control, and we expect prices of prescription pharmaceuticals to decline over the life of the product or as volumes increase.
We will not be able to successfully commercialize our current or future product candidates without establishing sales and marketing capabilities internally or through collaborators.
We currently have no sales and marketing staff. We may not be able to find suitable sales and marketing staff and collaborators for our products if and when they receive required approvals. We have no prior experience in the marketing, sale and distribution of pharmaceutical products, and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, effectively manage a geographically dispersed sales and marketing team, and maintain compliance by a marketing team with complex laws and regulations applicable to product marketing. Any collaborators may not be adequate or successful or could terminate or materially reduce the effort they direct to our products. The development of a marketing and sales capability will require significant expenditures, management resources and time. The cost of establishing such a sales force may exceed any potential product revenue, or our marketing and sales efforts may be unsuccessful. If we are unable to develop an internal marketing and sales capability in a timely fashion, or at all, or if we are unable to enter into a marketing and sales arrangement with a third party on acceptable terms, we may be unable to successfully develop and seek regulatory approval for our product candidates and/or effectively market and sell approved products, if any.
We face competition from other biotechnology and pharmaceutical companies and our financial condition and operations will suffer if we fail to compete effectively.
Technological competition is intense in the industry in which we operate. Competition comes from pharmaceutical companies, biotechnology companies, and universities, as well as companies that offer non-pharmaceutical solutions in the markets we may attempt to address with our products. Many of our competitors have substantially greater financial and technical resources; more extensive R&D capabilities; and greater marketing, distribution, production, and human resources than we do. Moreover, competitors may develop products more quickly than us and may obtain regulatory approval for such products more rapidly than we do. Products and processes which are more effective than those that we intend to develop may be developed by our competitors. R&D by others may render our product candidates non-competitive or obsolete.
Our product candidates may face competition sooner than expected.
We believe that DM199 could qualify for 12 years of data exclusivity in the United States under the Biologics Price Competition and Innovation Act of 2009 (BPCIA), which was enacted as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the ACA). Under the BPCIA, an application for a biosimilar product, or BLA, cannot be submitted to the FDA until four years, or if approved by the FDA, until 12 years, after the original brand product identified as the reference product is approved under a BLA. The BPCIA provides an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. This law is complex and is only beginning to be interpreted and implemented by the FDA. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for any of our product candidates that are biologics. There is also a risk that the U.S. Congress could repeal or amend the BPCIA to shorten this exclusivity period, potentially creating the opportunity for biosimilar competition sooner than anticipated after the expiration of our patent protection. Moreover, the extent to which a biosimilar, once approved, will be substituted for any reference product in a way that is similar to traditional generic substitution for non-biological products is not yet clear and will depend on a number of marketplace and regulatory factors that are still developing.
Even if, as we expect, our current or future product candidates are considered to be reference products eligible for 12 years of exclusivity under the BPCIA, another company could market competing products if the FDA approves a full BLA for such product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of the products. Moreover, an amendment or repeal of the BPCIA could result in a shorter exclusivity period for our product candidates, which could have a material adverse effect on our business.
Our relationships with customers and third-party payers will be subject to applicable fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payers will likely play a primary role in the recommendation, prescription and sale of any product candidates for which we receive marketing approval. Our future arrangements with third-party payers, customers and prescribing providers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, distribute and sell the products for which we receive marketing approval. Currently, applicable federal and state healthcare laws and regulations that may apply to our products and arrangements include the following:
|
● |
the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid. This statute may apply to our marketing practices, educational programs, pricing policies and relationships with healthcare providers in a position to prescribe or recommend our products. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation; |
|
● |
the federal False Claims Act imposes civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. The government also may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Pharmaceutical and other healthcare companies have been prosecuted under these laws for, among other things, allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws; |
|
● |
the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. There is an increasing trend for more criminal prosecutions and compliance enforcement activities for noncompliance with HIPAA as well as for data breaches involving protected individually identifiable health information. In the ordinary course of our business, we may receive protected health information from clinical study sites and clinicians. HIPAA restricts the use and disclosure of health information by most health care providers (Covered Entities) as well as by individuals and entities that perform various functions for or on behalf of Covered Entities (Business Associates). Depending on how we engage with health care providers in the development and commercialization of products, we may be deemed to be a Business Associate under HIPAA. Failure to comply with the HIPAA privacy and security standards may subject us to civil and criminal liability, and the cost of reporting and mitigating data breaches could be significant, all of which could have a material adverse effect on us and our operating results; |
|
● |
the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
|
● |
the federal Physician Payments Sunshine Act under the ACA requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare & Medicaid Services (CMS) information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and requires applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members and payments or other “transfers of value” to such physician owners. Effective January 2022, applicable manufacturers will also be required to collect and report information on payments or “transfers of value” to physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives; |
|
● |
analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures; |
|
● |
the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (PDMA) and its implementation regulations, as well as the Drug Supply Chain Security Act (DSCSA), which regulates the distribution of and tracing of prescription drugs and prescription drug samples at the federal level and sets minimum standards for the regulation of drug distributors by the states. The PDMA, its implementing regulations and state laws limit the distribution of prescription pharmaceutical product samples, and the DSCSA imposes requirements to ensure accountability in distribution and to identify and remove counterfeit and other illegitimate products from the market; and |
|
● |
the U.S. Foreign Corrupt Practices Act of 1977, as amended (FCPA), the U.S. domestic bribery statute contained in 18 U.S.C. §201 and the U.S. Travel Act. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. We are unable to predict what additional federal or state legislation or regulatory initiatives may be enacted in the future regarding our business or the healthcare industry in general, or what effect such legislation or regulations may have on us. Federal or state governments may impose additional restrictions or adopt interpretations of existing laws that could have a material adverse effect on us. Although we will endeavor to identify and comply with new laws and regulations and new interpretations of existing laws and regulations, it is possible that we may be unaware of new legal requirements or interpretations, which could result in our violation of these laws and/or regulations.
Laws, restrictions, and other regulatory measures are also imposed by anti-kickback prohibitions, fraud and abuse restrictions, and other healthcare laws and regulations in international jurisdictions, and in those jurisdictions, we face the same issues as in the United State regarding exposure to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm, and diminished profits and future earnings.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. Some of these laws may have safe harbors and/or exceptions that, if met, may protect our arrangements from liability. However, failure to meet any element of a safe harbor or exception may cause an arrangement to lose safe harbor/exception protection. There may not be safe harbors or exceptions for every potential financial arrangement we may enter into, and there can be no assurances that any of our arrangements or relationships will meet an otherwise applicable safe harbor or exception.
If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Any investigation of or action against us for violation of these laws, even if we successfully defend against it, would cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
We heavily rely on the capabilities and experience of our key executives and scientists, and the loss of any of them could affect our ability to develop our products.
We depend heavily on members of our management team and certain other key personnel, including in particular our clinical personnel. We also depend on our clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical, and regulatory personnel, particularly as we continue to expand our activities and seek regulatory approvals for clinical trials and eventually our DM199 product candidate. We enter into agreements with scientific and clinical collaborators and advisors, key opinion leaders, and academic partners in the ordinary course of our business. We also enter into agreements with physicians and institutions that will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officers or other key personnel could potentially harm our business, operating results, or financial condition.
We will likely need to expand our operations and increase the size of our company, and we may experience difficulties in managing growth.
As we advance DM199 and any future product candidates through preclinical testing and clinical studies, and develop our current or future product candidates, we will need to increase our product development, scientific, regulatory and compliance and administrative headcount to manage these programs. In addition, to continue to meet our obligations as a public company, we will likely need to increase our general and administrative capabilities. Our management, personnel and systems currently in place may not be adequate to support this future growth. Our need to effectively manage our operations, growth and various projects requires that we:
|
● |
successfully attract and recruit new employees with the expertise and experience we will require; |
|
● |
manage our clinical programs effectively, which we anticipate being conducted at numerous clinical sites; |
|
● |
develop a marketing, distribution and sales infrastructure if we seek to market our products directly; and |
|
● |
continue to improve our operational, manufacturing, quality assurance, financial and management controls, reporting systems and procedures. |
If we are unable to successfully manage this growth and increased complexity of operations, our business may be adversely affected.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failure to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing and reporting standards we have established, comply with federal and state health-care fraud and abuse laws and regulations, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions. Even if we are successful in defending against any such action, we would incur significant legal expenses in responding to and defending against it.
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We have in the past and may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations, or in-licensing one or more technologies or product candidates. Our ability to grow through acquisitions, collaborations and in-licenses will depend, in part, on the availability of suitable candidates at acceptable prices, terms and conditions, our ability to compete effectively for candidates, and the availability of capital and personnel resources to carry out such transactions. Acquisitions, collaborations and in-licenses involve numerous risks, including, among others:
|
● |
substantial cash expenditures; |
|
● |
adverse impact on overall profitability; |
|
● |
technology development risks; |
|
● |
potentially dilutive issuances of equity securities; |
|
● |
reallocation of amounts of capital from other initiatives; |
|
● |
incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
|
● |
write-off of significant amounts of goodwill, other intangible assets and/or long-lived assets as a result of deterioration in the performance of an acquired business, adverse market conditions, changes in the competitive landscape, changes in laws or regulations that restrict activities of an acquired business, or as a result of a variety of other circumstances; |
|
● |
difficulties in assimilating the information and financial systems, operations, processes and products of the acquired companies; |
|
● |
inability to effectively manage our expanded operations; |
|
● |
potential disputes regarding contingent consideration; |
|
● |
diverting our management’s attention away from other business concerns; |
|
● |
disruption to our existing operations and plans; |
|
● |
entering markets in which we have limited or no direct experience; |
|
● |
potential loss of our key employees or key employees of the acquired companies or businesses; |
|
● |
violation of confidentiality, intellectual property, and non-compete obligations or agreements by employees of an acquired business or lack of or inadequate formal intellectual property protection mechanisms in place at an acquired business; |
|
● |
failure by acquired businesses to comply with applicable international, federal and state regulatory standards; |
|
● |
infringement by acquired businesses of intellectual property rights of others; |
|
● |
inaccurate assessment of additional post-acquisition or business venture investments, undisclosed, contingent or other liabilities or problems, unanticipated costs associated with an acquisition, and an inability to recover or manage such liabilities and costs; and |
|
● |
incorrect estimates made in the accounting for acquisitions and incurrence of non-recurring charges. |
We cannot provide assurance that any acquisition, collaboration, or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the growth associated with some of these acquisitions, collaborations and in-licenses. We cannot provide assurance that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
Our DM199 product candidate or future product candidates may cause or have attributed to them undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial scope of their approved uses, or result in significant negative consequences following any marketing approval.
Undesirable side effects caused by our DM199 product candidate or any future product candidate or that may be identified as related to our product candidates by investigators conducting our clinical trials or even related to competing products in development that use a similar mechanism of action or act through a similar biological disease pathway could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities, potential product liability claims or a more restrictive label. Results of our trials could reveal unacceptable side effects or unexpected characteristics which could be attributed to our DM199 product candidate or any future product candidates. This may require longer and more extensive clinical development or we could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all of our targeted indications. Drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by any such products, a number of potentially significant negative consequences could result, including, among others:
|
● |
we may suspend marketing of, or withdraw or recall, such product; |
|
● |
regulatory authorities may withdraw approvals of such product; |
|
● |
regulatory authorities may require additional warnings on the label or otherwise seek to limit the scope of the approved uses reflected in the label of such product; |
|
● |
the FDA may require the use of or modification of a Risk Evaluation and Mitigation Strategy (REMS) or a comparable foreign regulatory authority may require the establishment or modification of a similar strategy that may, for instance, restrict distribution of our products and impose other implementation requirements on us; |
|
● |
regulatory authorities may require that we conduct post-marketing studies; |
|
● |
we could be sued and held liable for harm caused to patients; and |
|
● |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate or otherwise materially harm the commercial prospects for the product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We face the risk of product liability claims, which could exceed our insurance coverage and produce recalls, each of which could deplete our cash resources.
We are exposed to the risk of product liability claims alleging that use of our product candidates caused an injury or harm. These claims can arise at any point in the development, testing, manufacture, marketing, or sale of our product candidates and may be made directly by patients involved in clinical trials of our product candidates, by consumers or healthcare providers, or by individuals, organizations, or companies selling our products. Product liability claims can be expensive to defend, even if the product or product candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes increasingly expensive as a product candidate moves through the development pipeline to commercialization. To protect against potential product liability risks, we have AUD $20 million per occurrence and AUD $20 million aggregate clinical trial insurance for the REMEDY Phase II clinical trial in Australia and USD $5.0 million product liability insurance coverage. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available to us at a cost acceptable to us or at all. We may choose or find it necessary under our collaboration agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for damages resulting from a product liability claim could exceed the amount of our coverage, require us to pay a substantial monetary award from our own cash resources and have a material adverse effect on our business, financial condition, and results of operations. Moreover, a product recall, if required, could generate substantial negative publicity about our products and business, inhibit or prevent commercialization of other products and product candidates, or negatively impact existing or future collaborations.
If we are unable to maintain product liability insurance required by our third parties, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
Some of our license, clinical trials and other agreements with third parties require, and in the future will likely require, us to maintain product liability insurance in at least certain specified minimum amounts. If we cannot maintain acceptable amounts of coverage on commercially reasonable terms in accordance with the terms set forth in these agreements, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
A risk of product liability claims, and related negative publicity, is inherent in the development of human therapeutics and other products. Product liability insurance is expensive, its availability is limited, and it may not be offered on terms acceptable to us, or at all. The commercialization of our potential products could be inhibited or prevented by an inability to maintain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims. A product liability claim against us or the withdrawal of a product from the market could have a material adverse effect upon us and our financial condition.
A variety of risks are associated with operating our business internationally, which could materially adversely affect our business.
We currently conduct certain R&D operations in the United States and Australia. In the future, we expect to conduct certain clinical trials, and plan to seek regulatory approval of our product candidates, outside of the United States. Accordingly, we are subject to risks related to operating in foreign countries including, among others:
|
● |
different regulatory requirements for drug approvals in foreign countries; |
|
● |
different standards of care in various countries that could complicate the evaluation of our product candidates; |
|
● |
different United States and foreign drug import and export rules; |
|
● |
reduced protection for intellectual property rights in certain countries; |
|
● |
withdrawal from or revision to international trade policies or agreements and the imposition or increases in import and export licensing and other compliance requirements, customs duties and tariffs, import and export quotas and other trade restrictions, license obligations, and other non-tariff barriers to trade; |
|
● |
unexpected changes in tariffs, trade barriers, and regulatory requirements; |
|
● |
the imposition of U.S. or international sanctions against a country, company, person, or entity with whom we do business that would restrict or prohibit continued business with that country, company, person, or entity; |
|
● |
different reimbursement systems and different competitive drugs indicated to treat the indications for which our product candidates are being developed; |
|
● |
economic weakness, including inflation, or political instability in particular foreign economies and markets; |
|
● |
compliance with tax, employment, immigration, and labor laws for employees living or traveling abroad; |
|
● |
compliance with the Foreign Corrupt Practices Act and other anti-corruption and anti-bribery laws; |
|
● |
foreign taxes, including withholding of payroll taxes; |
|
● |
foreign currency exchange rate fluctuations, which could result in increased operating expenses and/or reduced revenue, and other obligations incident to doing business in another country; |
|
● |
difficulties in managing and staffing international operations and increases in infrastructure costs, including legal, tax, accounting, and information technology; |
|
● |
workforce uncertainty in countries where labor unrest is more common than in the United States; |
|
● |
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
|
● |
potential liability resulting from development work conducted by foreign partners; |
|
● |
transportation delays and interruptions; |
|
● |
business interruptions resulting from natural disasters or geopolitical actions, including war and terrorism, or systems failure, including cybersecurity breaches; and |
|
● |
compliance with evolving and expansive international data privacy laws, such as the European Union General Data Protection Regulation. |
Future legislation in the United States, Europe or other countries, and/or regulations and policies adopted by the FDA, the EMA or comparable regulatory authorities, may increase the time and cost required for us or our collaborator to conduct and complete clinical trials of our current or future product candidates.
The FDA and the EMA have each established regulations to govern the product development and approval process, as have other foreign regulatory authorities. The policies of the FDA, the EMA and other regulatory authorities may change. For example, in December 2016, the 21st Century Cures Act (Cures Act) was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and spur innovation, but not all of its provisions have yet been implemented. Additionally, in August 2017, the FDA issued final guidance setting forth its current thinking with respect to development programs and clinical trial designs for antibacterial drugs to treat serious bacterial diseases in patients with an unmet medical need. We cannot predict what if any effect the Cures Act or any existing or future guidance from the FDA or other regulatory authorities will have on the development of our product candidates.
Recently enacted and future legislation may increase the difficulty and cost for us and our future collaborators to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
Among policy makers and payers in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. For example, the ACA, which was enacted in the United States in March 2010, includes measures to change health care delivery, decrease the number of individuals without insurance, ensure access to certain basic health care services, and contain the rising cost of care. This healthcare reform movement, including the enactment of the ACA, has significantly changed health care financing by both governmental and private insurers in the United States. With respect to pharmaceutical manufacturers, the ACA increased the number of individuals with access to health care coverage, including prescription drug coverage, but it simultaneously imposed, among other things, increased liability for rebates and discounts owed to certain entities and government health care programs, fees for the manufacture or importation of certain branded drugs, and transparency reporting requirements under the Physician Payments Sunshine Act.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, as well as efforts by the current administration to repeal or replace certain aspects of the ACA. Since January 2017, two U.S. Presidential Executive Orders have been signed and other directives designed to delay the implementation of certain provisions of the ACA or otherwise remove some of the requirements for health insurance mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based individual shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year, which is commonly referred to as the “individual mandate.” Additionally, on January 22, 2018, the U.S. President signed a continuing resolution on appropriations for fiscal year 2018. This continuing resolution delayed implementation of the tax on certain high cost employer-sponsored insurance plans until January 1, 2022, provided a moratorium on the annual fee imposed on certain health insurance providers based on market share until January 1, 2020, and extended the moratorium on the medical device excise tax on non-exempt medical devices until January 1, 2020. Further, the Bipartisan Budget Act of 2018, or the “BBA,” among other things, amended the ACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.”
In addition to the ACA, other federal health reform measures have been proposed and adopted in the United States. For example, legislation has been enacted to reduce the level of reimbursement paid to providers under the Medicare program over time, as well as to phase in alternative payment models for provider services under the Medicare program with the goal of incentivizing the attainment of pre-defined quality measures. As these measures are not fully in effect, and since the U.S. Congress could intervene to prevent their full implementation, it is unclear how payment reductions or the introduction of the quality payment program will impact overall physician reimbursement under the Medicare program. It is also unclear if changes in Medicare payments to providers would impact such providers’ willingness to prescribe and administer our products, if approved. Further, there has been heightened governmental scrutiny over the manner in which companies set prices for their marketed products. For example, there have been several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and patient programs, and reform government program reimbursement methodologies for drug products.
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the price that we may receive for any product, if approved. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product candidates.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our current or future product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Our inability to maintain contractual relationships with physicians could have a negative impact on our research and development.
We maintain contractual relationships with respected physicians in hospitals and universities who assist us in the design of our clinical trials and interpretation of trial results. If we are unable to enter into and maintain these relationships, our ability to develop, obtain required regulatory approvals for, and market our product candidates could be adversely affected. In addition, it is possible that U.S. federal and state and international laws requiring us to disclose payments or other transfers of value, such as free gifts or meals, to surgeons and other healthcare providers could have a chilling effect on these relationships with individuals or entities that may, among other things, want to avoid public scrutiny of their financial relationships with us.
We conduct certain research and development operations through our Australian wholly-owned subsidiary. If we lose our ability to operate in Australia, or if our subsidiary is unable to receive the research and development incentive payment allowed by Australian regulations, our business and results of operations could suffer.
We maintain a wholly-owned Australian subsidiary, DiaMedica Australia Pty Ltd., to conduct various clinical activities for our product and development candidate in Australia. Due to the geographical distance and lack of employees currently in Australia, as well as our lack of experience operating in Australia, we may not be able to efficiently or successfully monitor, develop and commercialize our lead product candidate in Australia, including conducting clinical trials. Furthermore, we have no assurance that the results of any clinical trials that we conduct for our product candidate in Australia will be accepted by the FDA or foreign regulatory authorities for development and commercialization approvals.
In addition, current Australian tax regulations provide for a refundable R&D incentive payment equal to 43.5% of qualified expenditures. We received incentive payments of approximately USD $856,000 and USD $621,000 during 2019 and 2018, respectively, for research expenditures made during 2019 and 2018. If our subsidiary loses its ability to operate in Australia, or if we are ineligible or unable to receive the R&D incentive payment, or the Australian government significantly reduces or eliminates the incentive program, our business and results of operation may be adversely affected.
Fluctuations in insurance cost and availability could adversely affect our operating results or risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, property insurance, and workers’ compensation insurance. The costs of maintaining adequate insurance coverage, most notably directors’ and officers’, have increased significantly recently and may continue to do so in the future, thereby adversely affecting our operating results. If such costs continue to increase, we may be forced to accept lower coverage and higher deductibles, which would have an adverse effect on our risk management profile and inhibit our ability to recruit qualified directors and officers. In addition, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers.
Risks Related to Intellectual Property
If we are unable to adequately protect and enforce our intellectual property, our competitors may take advantage of our development efforts or acquired technology and compromise our prospects of marketing and selling our product candidates.
We believe that patents and other proprietary rights are key to our business. Our policy is to file patent applications to protect technology, inventions, and improvements that may be important to the development of our business. We also rely upon trade secrets, know-how, continuing technological innovations, and licensing opportunities to develop and maintain our competitive position. We plan to enforce our issued patents and our rights to proprietary information and technology. We review third-party patents and patent applications, both to refine our own patent strategy and to identify useful licensing opportunities.
Our success depends, in part, on our ability to secure and protect our intellectual property rights and to operate without infringing on the proprietary rights of others or having third parties circumvent the rights owned or licensed by us. We have a number of patents, patent applications and rights to patents related to our compounds, product candidates and technology, but we cannot be certain that they will be enforceable or provide adequate protection or that pending patent applications will result in issued patents.
To the extent that development, manufacturing, and testing of our product candidates is performed by third party contractors, such work is performed pursuant to fee for service contracts. Under the contracts, all intellectual property, technology know-how, and trade secrets arising under such agreements are our exclusive property and must be kept confidential by the contractors. It is not possible for us to be certain that we have obtained from the contractors all necessary rights to such technologies. Disputes may arise as to the scope of the contract or possible breach of contract. No assurance can be given that our contracts would be enforceable or would be upheld by a court.
The patent positions of pharmaceutical and biotechnology firms, ourselves included, are uncertain and involve complex questions of law and fact for which important legal issues remain unresolved. Therefore, it is not clear whether our pending patent applications will result in the issuance of patents or whether we will develop additional proprietary products which are patentable. Part of our strategy is based on our ability to secure a patent position to protect our technology. There is no assurance that we will be successful in this approach, and failure to secure patent protection may have a material adverse effect upon us and our financial condition. Also, we may fail in our attempt to commercialize products using currently patented or licensed technology without having to license additional patents. Moreover, it is not clear whether the patents issued or to be issued will provide us with any competitive advantages or if any such patents will be the target of challenges by third parties, whether the patents of others will interfere with our ability to market our products, or whether third parties will circumvent our patents by means of alternate processes. Furthermore, it is possible for others to develop products that have the same effect as our product candidates or technologies on an independent basis or to design around technologies patented by us. Patent applications relating to or affecting our business may have been filed by pharmaceutical or biotechnology companies or academic institutions. Such applications may conflict with our technologies or patent applications and such conflict could reduce the scope of patent protection that we could otherwise obtain or even lead to the rejection of our patent applications. There is no assurance that we can enter into licensing arrangements on commercially reasonable terms or develop or obtain alternative technology in respect of patents issued to third parties that incidentally cover our products or production technologies. Any inability to secure licenses or alternative technology could result in delays in the introduction of some of our product candidates or even lead to us being prevented from pursuing the development, manufacture or sale of certain products. Moreover, we could potentially incur substantial legal costs in defending legal actions that allege patent infringement, or by initiating patent infringement suits against others. It is not possible for us to be certain that we are the creator of inventions covered by pending patent applications or that we were the first to invent or file patent applications for any such inventions. While we have used commercially reasonable efforts to obtain assignments of intellectual property from all individuals who may have created materials on our behalf (including with respect to inventions covered by our patent and pending patent applications), it is not possible for us to be certain that we have obtained all necessary rights to such materials. No assurance can be given that our patents, if issued, would be upheld by a court, or that a competitor’s technology or product would be found to infringe on our patents. Moreover, much of our technology know-how that is not patentable may constitute trade secrets. Therefore, we require our employees, consultants, advisors and collaborators to enter into confidentiality agreements either as stand-alone agreements or as part of their employment or consulting contracts. However, no assurance can be given that such agreements will provide meaningful protection of our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of confidential information. Also, while we have used commercially reasonable efforts to obtain executed copies of such agreements from all employees, consultants, advisors and collaborators, no assurance can be given that executed copies of all such agreements have been obtained.
We may require additional third-party licenses to effectively develop and manufacture our product candidates and are currently unable to predict the availability or cost of such licenses.
A substantial number of patents have already been issued to other biotechnology and pharmaceutical companies. To the extent that valid third-party patent rights cover our product candidates, we or our strategic collaborators would be required to seek licenses from the holders of these patents in order to manufacture, use, or sell these product candidates, and payments under them would reduce our profits from these product candidates. We are currently unable to predict the extent to which we may wish or be required to acquire rights under such patents, the availability and cost of acquiring such rights, and whether a license to such patents will be available on acceptable terms, or at all. There may be patents in the United States or in foreign countries or patents issued in the future that are unavailable to license on acceptable terms. Our inability to obtain such licenses may hinder or eliminate our ability to develop, manufacture and market our product candidates.
Changes in patent law and its interpretation could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology and pharmaceutical companies, our success is heavily dependent on intellectual property rights, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves technological and legal complexity, and obtaining and enforcing biopharmaceutical patents is costly, time consuming, and inherently uncertain. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office (USPTO), the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future. Changes in either the patent laws or interpretation of the patent laws in the United States or other countries could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents.
Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could, therefore, be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our or our licensor’s patents or patent applications.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent in USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, and similar legislative, judicial, and administrative bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future.
Litigation regarding patents, patent applications, and other proprietary rights may be expensive, time consuming and cause delays in the development and manufacturing of our product candidates.
Third parties may claim that we are using their proprietary information without authorization. Third parties may also have or obtain patents and may claim that technologies licensed to or used by us infringe their patents. If we are required to defend patent infringement actions brought by third parties, or if we sue to protect our own patent rights or otherwise to protect our proprietary information and to prevent its disclosure, we may be required to pay substantial litigation costs and managerial attention may be diverted from business operations even if the outcome is in our favor. In addition, any legal action that seeks damages or an injunction to stop us from carrying on our commercial activities relating to the affected technologies could subject us to monetary liability (including treble damages and attorneys’ fees if we are found to have willfully infringed) and require us or any third-party licensors to obtain a license to continue to use the affected technologies. We cannot predict whether we would prevail in any of these types of actions or that any required license would be available on commercially acceptable terms or at all. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources.
Competitors may infringe our patents or other intellectual property. If we were to initiate legal proceedings against a third party to enforce a patent covering our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Moreover, similar challenges may be made by third parties outside the context of litigation, e.g., via administrative proceedings such as post grant or inter partes review in the United States or via oppositions or other similar proceedings in other countries/regions.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Our defense of litigation, validity or enforceability, interference or derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation or such other proceedings could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the market price of our common shares.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are a party to a license agreement relating to an expression system and cell line for use in the production of DM199 or any human KLK1, and we may need to obtain additional licenses from others to advance our R&D activities or allow the commercialization of DM199 or any other product candidates we may identify and pursue. Future license agreements may impose various development, diligence, commercialization and other obligations on us. If any of our in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties may gain access to technologies that are material to our business, and we may be required to cease our development and commercialization of DM199 or other product candidates that we may identify or to seek alternative manufacturing methods. However, suitable alternatives may not be available or the development of suitable alternatives may result in a significant delay in our commercialization of DM199. Any of the foregoing could have a material adverse effect on our competitive position, business, financial condition, results of operations and prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including, among others:
|
● |
the scope of rights granted under the license agreement and other interpretation-related issues; |
|
● |
the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
|
● |
the sublicensing of patent and other rights under our collaborative development relationships; |
|
● |
our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
|
● |
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
|
● |
the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third parties to develop our products, we must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, employment or consulting agreements, or other similar agreements with our collaborators, advisors, employees, and consultants prior to beginning research or disclosing proprietary information. These agreements typically restrict the ability of our collaborators, advisors, employees, and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. We also conduct joint R&D programs which may require us to share trade secrets under the terms of R&D collaboration or similar agreements. However, we cannot be certain that such agreements have been entered into with all relevant parties. Moreover, despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development, or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. Trade secrets can be difficult to protect. If the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secrets. A competitor’s discovery of our trade secrets may impair our competitive position and could have a material adverse effect on our business and financial condition.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employees’ former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Common Shares and this Offering
Our management will have broad discretion and flexibility as to how to use the net proceeds from this offering and may use the net proceeds in ways with which you disagree or which may not prove effective.
We currently intend to use the net proceeds from this offering as discussed under “Use of Proceeds” in this prospectus supplement. We have not allocated specific amounts of the net proceeds from this offering for any of the purposes set forth in that section. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
Purchasers of common shares in this offering will experience immediate and substantial dilution in the book value of their investment. You may experience further dilution upon exercise of our outstanding options and warrants.
The public offering price per common share in this offering is higher than the net tangible book value per common share before giving effect to this offering. Accordingly, if you purchase common shares in this offering, you will incur immediate substantial dilution of approximately $2.77 per share, representing the difference between the public offering price of $4.00 per common share, and our as adjusted net tangible book value per share as of September 30, 2019. In addition, if outstanding options or warrants are exercised, you could experience further dilution. For a further description of the dilution that you will experience immediately after this offering, see the section in this prospectus entitled “Dilution.”
Our common share price has been volatile in recent years and may continue to be volatile.
Our common shares trade on The Nasdaq Capital Market under the trading symbol “DMAC.” A number of factors could influence the volatility in the trading price of our common shares, including changes in the economy or in the financial markets, industry related developments, and the impact of material events and changes in our operations. Our quarterly losses may vary because of expenses we incur related to future research including the timing of costs for manufacturing and initiating and completing preclinical and clinical trials. Each of these factors could lead to increased volatility in the market price of our common shares. In addition, the market prices of the securities of our competitors may also lead to fluctuations in the trading price of our common shares. As a result of this volatility, you may not be able to sell your common shares at or above the public offering price.
We do not have a very active trading market for our common shares, and one may never develop.
We do not have a very active trading market for our common shares, and one may never develop, even after this offering. Although we anticipate a more active trading market for our shares will develop after this offering, we can give no assurance that this will occur or that an active trading market will be sustained following this offering. If an active market for our common shares does not develop, it may be difficult for you to sell shares you purchase in this offering at a favorable price or at all.
We have never paid dividends and do not expect to do so in the foreseeable future.
We have not declared or paid any cash dividends on our common shares to date. The payment of dividends in the future will be dependent on our earnings and financial condition and on such other factors as our Board of Directors considers appropriate. Unless and until we pay dividends, shareholders may not receive a return on their shares. There is no present intention by our Board of Directors to pay dividends on our common shares. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, appreciation, if any, in the market price of our common shares will be your sole source of gain for the foreseeable future.
We may issue additional common shares resulting in share ownership dilution.
Future dilution may occur due to additional future equity financing events by us. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. In addition, if outstanding options, warrants, or deferred share units are exercised or otherwise converted into our common shares, our shareholders will experience additional dilution.
If there are substantial sales of our common shares, the market price of our common shares could decline.
Sales of substantial numbers of our common shares could cause a decline in the market price of our common shares. Any sales by existing shareholders or holders who exercise their warrants or stock options may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common shares.
We could be subject to securities class action litigation, which is expensive and could divert management attention.
In the past, securities class action litigation has often been brought against a company following a decline or increase in the market price of its securities or certain significant business transactions. We may become involved in this type of litigation in the future. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and our resources, which could harm our business.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, the market stock of our common shares and trading volume could decline.
The trading market for our common shares in the United States after this offering will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the market price of our common shares or trading volume to decline.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common shares less attractive to our shareholders and other investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. We may remain an emerging growth company until the last day of the fiscal year following the fifth anniversary of our first sale of common shares pursuant to a registration statement under the Securities Act of 1933, as amended, or the “Securities Act,” until such earlier time as we have more than $1.07 billion in annual revenue, the market value of our common shares held by non-affiliates is more than $700 million or we issue more than $1 billion of non-convertible debt over a three-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (Section 404) not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Our shareholders and other investors may find our common shares less attractive as a result of our reliance on these exemptions. If some of our shareholders or other investors find our common shares less attractive as a result, there may be a less active trading market for our common shares, and the trading price of our common shares may be more volatile.
In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised financial accounting standards. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we have determined to opt out of such extended transition period and, as a result, we will comply with new or revised financial accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised financial accounting standards is irrevocable.
Our shareholders and other investors may find our common shares less attractive as a result of our reliance on these exemptions. If some of our shareholders or other investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and the trading price of our common shares may be more volatile.
Our inability to comply with Nasdaq’s continued listing requirements could result in our common shares being delisted, which could affect the market price and liquidity of our common shares and reduce our ability to raise capital.
Upon completion of this offering, we will be required to meet certain qualitative and financial tests to maintain the listing of our common shares on The Nasdaq Capital Market. If we do not maintain compliance with Nasdaq’s continued listing requirements within specified periods and subject to permitted extensions, our common shares may be recommended for delisting (subject to any appeal we would file). No assurance can be provided that we will comply with these continued listing requirements. If our common shares were delisted, it could be more difficult to buy or sell our common shares and to obtain accurate quotations, and the price of our common shares could suffer a material decline. Delisting would also impair our ability to raise capital.
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares. As a result of our limited administrative staffing levels, internal controls which rely on segregation of duties in many cases are not possible. Due to resource constraints and the present stage of our development, we do not have sufficient size and scale to warrant the hiring of additional staff to address this potential weakness at this time. To help mitigate the impact of this, we are highly reliant on the performance of compensating procedures and senior management’s review and approval. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with GAAP, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of shareholder or other investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our common shares.
Pursuant to Section 404 of the Sarbanes-Oxley Act, we will be required to furnish a report by our management on our internal control over financial reporting, and after we are no longer an emerging growth company, we will be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will have to engage in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. There is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Risks Related to Our Jurisdiction of Organization
It may be difficult for non-Canadian shareholders or other investors to obtain and enforce judgments against us because of our organization as a British Columbia corporation and presence.
We are a corporation governed under the British Columbia Business Corporations Act. Two of our directors and all or a substantial portion of their assets, and a portion of our assets, are located outside the United States. Consequently, it may be difficult for holders of our securities who reside in the United States to effect service within the United States upon those directors and experts who are not residents of the United States. It may also be difficult for holders of our securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers, and experts under the United States federal securities laws. Our shareholders and other investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or such directors, officers, or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state or jurisdiction of the United States, or (ii) would enforce, in original actions, liabilities against us or such directors, officers, or experts predicated upon the United States federal securities laws or any securities or “blue sky” laws of any state or jurisdiction of the United States. In addition, the protections afforded by Canadian securities laws may not be available to our shareholders or other investors in the United States.
Canadian laws differ from the laws in effect in the United States and may afford less protection to holders of our securities.
We are a corporation governed under and are subject to the BCBCA and applicable Canadian securities laws as a Canadian reporting issuer, which laws may differ from those governing a company formed under the laws of a United States jurisdiction. The provisions under the BCBCA and other relevant laws may affect the rights of shareholders differently than those of a company governed by the laws of a United States jurisdiction, and may, together with our notice of articles and articles, have the effect of delaying, deferring or discouraging another party from acquiring control of our company by means of a tender offer, a proxy contest or otherwise, or may affect the price an acquiring party would be willing to offer in such an instance.
We are governed by the corporate laws in British Columbia, Canada which in some cases have a different effect on shareholders than the corporate laws in Delaware, United States.
The material differences between the BCBCA as compared to the Delaware General Corporation Law (DGCL), which may be of most interest to shareholders include the following: (i) for material corporate transactions (such as mergers and amalgamations, other extraordinary corporate transactions, amendments to our notice of articles), the BCBCA generally requires two-thirds majority vote by shareholders, whereas DGCL generally only requires a majority vote of shareholders for similar material corporate transactions; (ii) under the BCBCA, a holder of 5% or more of our common shares can requisition a special meeting at which any matters that can be voted on at our annual meeting can be considered, whereas the DGCL does not give this right; (iii) our Articles require two-thirds majority vote by shareholders to pass a resolution for one or more directors to be removed, whereas DGCL only requires the affirmative vote of a majority of the shareholders; however, many public company charters limit removal of directors to a removal for cause; and (iv) our Articles may be amended by resolution of our directors to alter our authorized share structure, including to (a) consolidate or subdivide any of our shares and (b) create additional classes or series of shares, whereas under DGCL, a majority vote by shareholders is generally required to amend a corporation’s certificate of incorporation and a separate class vote may be required to authorize alterations to a corporation’s authorized share structure. We cannot predict if investors will find our common shares less attractive because of these material differences. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares, and our share price may be more volatile.
Our shareholder rights plan may delay or prevent an acquisition of us that shareholders may consider favorable or may prevent efforts by our shareholders to change our directors or our management, which could decrease the value of your common shares.
On December 21, 2017, our shareholders approved the renewal of a shareholder rights plan agreement through the annual general meeting to be held by DiaMedica in 2020. The shareholder rights plan is designed to provide adequate time for our Board of Directors and shareholders to assess an unsolicited takeover bid for our company, to provide our Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The shareholder rights plan is set to expire at the close of our annual meeting of shareholders in 2020. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of our outstanding common shares without complying with the “permitted bid” provisions of the plan or without approval of our Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time. Under the plan, a “permitted bid” is a bid made to all holders of our common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
While we believe our rights plan enables our Board of Directors to help ensure that our shareholders are not deprived of the opportunity to realize the full and fair value of their investments, the rights plan may inhibit a change in control of our company by a third party in a transaction not approved by our Board of Directors. If a change in control is inhibited or delayed in this manner, it may adversely affect the market price of our common shares.
We may be classified as a “passive foreign investment company,” which may have adverse U.S. federal income tax consequences for U.S. shareholders.
Generally, for any taxable year in which 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our common shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company (PFIC) for U.S. federal income tax purposes. The average percentage of a corporation’s assets that produce or are held for the production of passive income generally is determined on the basis of the fair market value of the corporation’s assets at the end of each quarter (which may be determined in part by the market value of our common shares, which is subject to change).
The tests for determining PFIC status for any taxable year are dependent upon a number of factors, some of which are beyond our control, including the value of our assets, the market price of our common shares, and the amount and type of our gross income. Based on these tests, (i) we believe that we were a PFIC for the taxable year ended December 31, 2016, and (ii) we do not believe that we were a PFIC for the taxable years ended December 31, 2019, 2018 and 2017. Our status as a PFIC is a fact-intensive determination made for each taxable year, and we cannot provide any assurance regarding our PFIC status for the taxable year ending December 31, 2020 or for subsequent taxable years. U.S. shareholders who own our common shares for any period during which we are a PFIC will be required to file IRS Form 8621 for each tax year during which they hold our common shares.
If we are a PFIC for any year during a non-corporate U.S. shareholder’s holding period of our common shares, and the U.S. shareholder does not make a “qualified electing fund” election (QEF election) or a “mark-to-market” election, both as described below, then such non-corporate U.S. shareholder generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called “excess distribution” received on our common shares, as ordinary income, rather than as capital gain, and the preferential tax rate applicable to dividends received on our common shares would not be available. This income generally would be allocated over a U.S. shareholder’s holding period with respect to our common shares, and the amount allocated to prior years will be subject to tax at the highest tax rate in effect for that year, and an interest charge would be imposed on the amount of deferred tax on the income allocated to prior taxable years. Pursuant to the specific provisions of the PFIC rules, a taxpayer may realize gain on the disposition of common shares if (i) the securities are disposed of by a holder whose securities are attributed to the U.S. shareholder, or (ii) the securities are pledged as security for a loan, transferred by gift or death, or are subject to certain corporate distributions. Additionally, if we are a PFIC, a U.S. shareholder who acquires our common shares from a decedent would be denied normally available step-up in tax basis for our common shares to fair market value at the date of death but instead would have a tax basis equal to the lower of the fair market value of such common shares or the decedent’s tax basis in such common shares.
A U.S. shareholder may avoid these adverse tax consequences by making a timely and effective QEF election. A U.S. shareholder who makes a QEF election generally must report, on a current basis, its share of our ordinary earnings and net capital gains, whether or not we distribute any amounts to our shareholders, and would be required to comply with specified information reporting requirements. Any gain subsequently recognized upon the sale by that U.S. shareholder of the common shares generally would be taxed as capital gain and the denial of the basis step-up at death described above would not apply. The QEF election is available only if the company characterized as a PFIC provides a U.S. shareholder with certain information regarding its earnings and capital gains, as required under applicable U.S. Treasury regulations. We intend to provide all information and documentation that a U.S. shareholder making a QEF election is required to obtain for U.S. federal income tax purposes (e.g., the U.S. shareholder’s pro rata share of ordinary income and net capital gain, and a “PFIC Annual Information Statement” as described in applicable U.S. Treasury regulations).
As an alternative to a QEF election, a U.S. shareholder may also mitigate the adverse tax consequences of PFIC status by timely making a “mark-to-market” election. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the increase in the fair market value of the common shares and deduct from gross income the decrease in the value of such shares during each of its taxable years. Losses would be allowed only to the extent of the net mark-to-market gain accrued under the election. If a mark-to-market election with respect to our common shares is in effect on the date of a U.S. shareholder’s death, the tax basis of the common shares in the hands of a U.S. shareholder who acquired them from a decedent will be the lesser of the decedent’s tax basis or the fair market value of the common shares. A mark-to-market election may be made and maintained only if our common shares are regularly traded on a qualified exchange, including Nasdaq. Whether our common shares are regularly traded on a qualified exchange is an annual determination based on facts that, in part, are beyond our control. Accordingly, a U.S. shareholder might not be eligible to make a mark-to-market election to mitigate the adverse tax consequences if we are characterized as a PFIC.
Certain economic risks are inherent in making either a QEF election or a mark-to-market election. If a QEF election is made, it is possible that a small but significant amount of earned income will be reported to a U.S. shareholder as taxable income as long as the company invests its cash reserves, and income taxes will be due and payable on such an amount. A U.S. shareholder of our common shares may pay tax on such “phantom” income, i.e., income reported to it pursuant to the QEF election, but not actually received. There is no assurance that any distribution or profitable sale will ever be made regarding our common shares, so the tax liability may result in a net economic loss. A mark-to-market election may result in significant share price gains in one year causing a significant income tax liability. This gain may be offset in another year by significant losses. If a mark-to-market election is made, this highly variable tax gain or loss may result in substantial and unpredictable changes in taxable income. The amount included in income under a mark-to-market election may be substantially greater than the amount included under a QEF election. Both the QEF and mark-to-market elections are binding on the U.S. shareholder for all subsequent years that the U.S. shareholder owns our stock unless permission to revoke the election is granted by the IRS.
Although we generally will continue to be treated as a PFIC as to any U.S. shareholder if we are a PFIC for any year during a U.S. shareholder’s holding period, if we cease to satisfy the requirements for PFIC classification, the U.S. shareholder may avoid PFIC classification for subsequent years if the U.S. shareholder elects to make a so-called “purging election,” by recognizing income based on the unrealized appreciation in the common shares through the close of the tax year in which we cease to be a PFIC.
RULES RELATING TO A PFIC ARE VERY COMPLEX. YOU SHOULD CONSULT YOUR TAX ADVISER CONCERNING THE RELATIVE MERITS AND THE ECONOMIC AND TAX IMPACT OF PFIC RULES TO YOUR INVESTMENT IN OUR COMMON SHARES AS A NON-ELECTING U.S. SHAREHOLDER, A U.S. SHAREHOLDER MAKING A QEF ELECTION, OR A U.S. SHAREHOLDER MAKING A MARK-TO-MARKET ELECTION.
Should we be classified as a PFIC during a U.S. shareholder’s holding period for our common shares, each such U.S. shareholder should consult their own tax advisors with respect to the possibility of making these elections and the U.S. federal income tax consequences of the acquisition, ownership and disposition of our common shares. In addition, the possibility of us being classified as a PFIC may deter certain U.S. investors from purchasing our common shares, which could have an adverse impact on the market price of our common shares and our ability to raise additional financing by selling equity securities, including our common shares.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this prospectus supplement and the related prospectus supplement that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology, and the use of future dates. The forward-looking statements in or incorporated by reference into this prospectus supplement or the related prospectus supplement may include, among other things, statements about:
|
● |
our plans to develop, obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of CKD and AIS and our expectations regarding the benefits of our DM199 product candidate; |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain required regulatory approvals of our DM199 product candidate for CKD and AIS; |
|
● |
the perceived benefits of our DM199 product candidate over existing treatment options for CKD and AIS; |
|
● |
the potential size of the markets for our DM199 product candidate and our ability to serve those markets; |
|
● |
the rate and degree of market acceptance, both in the United States and internationally, of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for and commercialize our DM199 product candidate for CKD and AIS; |
|
● |
the success, cost and timing of planned clinical trials, as well as our reliance on collaboration with third parties to conduct our clinical trials; |
|
● |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
● |
expectations regarding federal, state, and foreign regulatory requirements and developments, such as potential United States Food and Drug Administration (FDA) regulation of our DM199 product candidate for CKD and AIS; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain funding for our operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for our DM199 product candidate for CKD and AIS; |
|
● |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; and |
|
● |
our anticipated use of the net proceeds from our December 2018 initial public offering in the United States and this offering. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under “Risk Factors” in this prospectus supplement and in the documents we incorporate by reference into this prospectus supplement. Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Except as required by law, including the securities laws of the United States, we do not intend to update any forward-looking statements to conform these statements to actual results or to changes in our expectations.
USE OF PROCEEDS
We estimate that the net proceeds from the sale of our common shares in this offering will be approximately $7.7 million, based on a public offering price of $4.00 per share, after deducting the underwriting discounts and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering to continue our clinical and product development activities and for other working capital and general corporate purposes.
Pending the uses described above, we may deposit the proceeds in our non-interest bearing checking account, interest bearing money market fund or invest them in short-term or marketable securities until we use them for their stated purpose.
DIVIDEND POLICY
We have not declared or paid any cash dividends on our common shares since our inception. We currently intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments, provisions of applicable law and other factors the Board deems relevant.
CAPITALIZATION
The following table sets forth our cash and capitalization as of September 30, 2019 (a) on an actual basis, and (b) on an as adjusted basis to give effect to the sale by us of common shares in this offering at the public offering price of $4.00 per share, after deducting the underwriting discount and estimated offering expenses payable by us.
This table should be read with “Use of Proceeds” in this prospectus supplement, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included in our Quarterly Report on Form 10-Q for the nine months ended September 30, 2019, which is incorporated by reference into this prospectus supplement.
|
As of September 30, 2019 |
||||||||
|
(unaudited) (in thousands) |
||||||||
|
Actual |
As Adjusted |
|||||||
|
Cash, cash equivalents and marketable securities |
$ | 9,744 | $ | 17,443 | ||||
|
Capitalization: |
||||||||
|
Shareholders’ equity: |
||||||||
|
Common shares, no par value; unlimited authorized; 12,006,874 actual issued and outstanding; as adjusted for this offering |
$ | – | $ | – | ||||
|
Additional paid-in capital |
63,831 | 71,530 | ||||||
|
Accumulated other comprehensive income |
6 | 6 | ||||||
|
Accumulated deficit |
(54,137 | ) | (54,137 | ) | ||||
|
Total shareholders’ equity |
$ | 9,700 | $ | 17,399 | ||||
|
Total capitalization |
$ | 11,039 | $ | 18,738 | ||||
The number of our common shares to be outstanding immediately after this offering, as shown above, is based on 12,006,874 common shares issued and outstanding as of September 30, 2019, and excludes as of that date:
|
● |
1,012,563 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.44 per share; |
|
● |
624,568 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.10 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
627,325 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,372,675 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
DILUTION
If you invest in our common shares in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per common share and the as adjusted net tangible book value per share of our common shares immediately after this offering. Net tangible book value per common share is determined at any date by subtracting our total liabilities from the amount of our total tangible assets (total assets less intangible assets) and dividing the difference by the number of our common shares deemed to be outstanding at that date.
Our net tangible book value as of September 30, 2019 was approximately $9.7 million, or $0.81 per share, based on 12,006,874 common shares outstanding as of September 30, 2019. After giving effect to our sale of common shares in this offering at the public offering price of $4.00 per share, after deducting estimated underwriting discounts and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2019 would have been approximately $17.4 million, or $1.23 per share. This amount represents an immediate increase in net tangible book value of $0.42 per common share to existing shareholders and an immediate dilution in net tangible book value of $2.77 per common share to new investors purchasing common shares in this offering.
The following table illustrates this dilution on a per share basis:
|
Public offering price per share |
$ | 4.00 | ||||||
|
Net tangible book value per share as of September 30, 2019 |
$ | 0.81 | ||||||
|
Increase per share attributable to this offering |
$ | 0.42 | ||||||
|
Net tangible book value per share after this offering |
$ | 1.23 | ||||||
|
Dilution per share to new investors participating in this offering |
$ | 2.77 |
The number of our common shares to be outstanding immediately after this offering, as shown above, is based on 12,006,874 common shares issued and outstanding as of September 30, 2019, and excludes as of that date:
|
● |
1,012,563 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.44 per share; |
|
● |
624,568 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.10 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
627,325 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,372,675 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
To the extent that any of these common shares are issued upon exercise of stock options or warrants, there may be further dilution to new public investors. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing our common shares or other securities exercisable or exchangeable for, or convertible into, our common shares, your ownership will be further diluted.
DESCRIPTION OF COMMON SHARES
General
The following is a summary of the material terms of our common shares, as well as other material terms of our Notice of Articles and Articles and certain provisions of the British Columbia Business Corporations Act (BCBCA). References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value. This summary does not purport to be complete and is qualified in its entirety by the provisions of our Notice of Articles and Articles, which are included as exhibits to the registration statement of which this prospectus forms a part. For more information on how you can obtain our Notice of Articles and Articles, see the heading “Where You Can Find Additional Information.”
Authorized Share Capital
We have an authorized share capital consisting of an unlimited number of common shares, no par value per share.
Certain Rights of the Common Shares
Dividends
Holders of our common shares are entitled to share pro rata in such dividends as may be declared by our Board of Directors. Pursuant to the provisions of the BCBCA, we may not declare or pay a dividend if there are reasonable grounds for believing that we are, or would after the payment be, unable to pay our liabilities as they become due in the ordinary course of business. We may pay a dividend by issuing fully paid shares, bonds, debentures or other of our securities or in property (including money).
Liquidation, Dissolution or Winding-Up
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company or any other distribution of our assets among our shareholders for the purpose of winding-up our affairs, holders of common shares are entitled to share pro rata in our assets available for distribution after we pay our creditors.
Voting Rights and Shareholders’ Meetings
Holders of our common shares are entitled to receive notice of and to attend and vote at all meetings of our shareholders. Each holder of our common shares is entitled to one vote, either in person or by proxy, on all matters submitted to shareholders.
Our Board of Directors must call an annual general meeting of shareholders to be held not later than 15 months after the last preceding annual general meeting of shareholders but no later than six months after the end of our preceding financial year end and may, at any time, call a special meeting of shareholders. Under our Articles, a meeting of our shareholders may be held anywhere in or outside of British Columbia, as determined by the Board of Directors. For purposes of determining the shareholders who are entitled to receive notice of or to vote at a meeting of shareholders, the Board of Directors may, in accordance with National Instrument 54-101 - Communications with Beneficial Owners of Securities of a Reporting Issuer of the Canadian Securities Administrators, fix in advance a date as the record date for that determination of shareholders, but that record date may not be more than 60 days or less than 30 days before the date on which the meeting is to be held.
Our Articles provide that notice of the time and place of a meeting of shareholders must be sent to each shareholder entitled to vote at the meeting, each director and to our auditors, not more than 50 days and not less than 21 days prior to the meeting. Under our Articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding not less than one-third (33 1/3) of the issued common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting. A shareholder may participate in a meeting by means of telephone or other communication medium that permits all persons participating in the meeting to communicate with each other during the meeting.
In the case of joint shareholders, one of the holders present at a meeting, either personally or by proxy, may, in the absence of the other holder(s) of the shares, vote the shares. If two or more joint shareholders are present, personally or by proxy, then only the vote of the joint shareholder present whose name stands first on the central securities register in respect of the share will be counted.
No Preemption Rights; Limited Restrictions on Directors’ Authority to Issue Common Shares
Existing holders of our common shares have no rights of preemption or first refusal under our Articles or the BCBCA with respect to future issuances of our common shares. The common shares do not have conversion rights, are not subject to redemption and do not have the benefit of any sinking fund provisions. Subject to the rules and policies of The Nasdaq Stock Market and applicable corporate and securities laws, our Board of Directors has the authority to issue additional common shares.
Amendments to Articles
The Articles and the BCBCA govern the rights of holders of our common shares.
Subject to the BCBCA, unless an alteration to the Company’s Notice of Articles would be required, our directors can authorize the alteration of our Articles to, among other things, create additional classes or series of shares or, if none of the shares of a class or series are allotted or issued, eliminate that class or series of shares.
Subject to the BCBCA, our shareholders can authorize the alteration of our Articles and Notice of Articles to create or vary the rights or restrictions attached to any class of our shares by passing an ordinary resolution at a duly convened meeting of shareholders. An alteration to the Company’s Notice of Articles will not be effective until the notice of alteration is filed with the registrar pursuant to the BCBCA. An alteration to the Company’s Articles, which is not an alteration to the Company’s Notice of Articles, will be effective on the date and time that the resolution is received for deposit at the Company’s records office.
Fundamental Changes
Pursuant to the BCBCA, we may not effect any of the following fundamental changes without the consent of the holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and separately as a class at a duly convened meeting of our shareholders:
|
● |
any proposed amalgamation (consolidation or merger) involving our company in respect of which the BCBCA requires that the approval of our shareholders be obtained; |
|
● |
any proposed plan of arrangement pursuant to the BCBCA involving our company in respect of which the BCBCA or any order issued by an applicable court requires that the approval of our shareholders be obtained; |
|
● |
any proposed sale, lease or exchange of all or substantially all of our undertaking; and |
|
● |
any voluntary liquidation of our company. |
Election and Removal of Directors
At each annual general meeting of shareholders, our shareholders are required to elect directors to hold office for a term expiring not later than the close of the next annual general meeting of shareholders. Our Board of Directors may fill vacancies among the Board. Our directors may also, between annual general meetings of our shareholders, appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third (1/3) of the number of directors who held office at the expiration of the last meeting of shareholders.
Since shareholders do not have cumulative voting rights, holders of more than 50% of our outstanding common shares can elect all of our directors if they choose to do so. In such event, holders of the remaining shares will be unable to elect any director.
Under the BCBCA, a public company must have a minimum of three directors, who are not required to be resident Canadians.
Under the BCBCA, a director may be removed by shareholders by special resolution unless the Articles provide for a lower approval level. The Articles allow shareholders to remove directors by a special resolution if approved by holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and voting separately as a class at a duly convened meeting of our shareholders.
Registration Rights
We have not granted any rights to have our common shares or other securities registered under the United States Securities Act of 1933, as amended (Securities Act).
Listing
Our common shares are listed and trade in the United States on The Nasdaq Capital Market under the trading symbol “DMAC.”
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
Limitation of Liability and Indemnification Matters
Our Articles provide that we will indemnify our directors, former directors, his or her heirs and legal personal representatives and other individuals as we may determine against all eligible penalties to which such person is or may be liable to the fullest extent permitted by British Columbia law. We will pay all expenses actually and reasonably incurred by such person, either as such expenses are incurred in advance of the final disposition of an eligible proceeding or after the final disposition of an eligible proceeding. British Columbia law provides that a company must not indemnify its directors if any of the following circumstances apply:
|
● |
if the indemnity or payment is made under an earlier agreement to indemnify or pay expenses and, at the time that the agreement to indemnify or pay expenses was made, the company was prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if the indemnity or payment is made otherwise than under an earlier agreement to indemnify or pay expenses and, at the time that the indemnity or payment is made, the company is prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if, in relation to the subject matter of the relevant proceeding, the director did not act honestly and in good faith with a view to the best interests of the company or the associated corporation, as the case may be, with such associated corporation being an affiliate of the company or a partnership, trust, joint venture or other unincorporated entity in which the director served in the capacity as a director or a position equivalent to that thereof, at the request of the company; or |
|
● |
in the case of the relevant proceeding other than a civil proceeding, if the director did not have reasonable grounds for believing that the director’s conduct in respect of which the proceeding was brought was lawful. |
Notwithstanding any of the above prohibitions, the company or a director may apply to court for an order that the company must indemnify the director for any liability or expenses incurred by the director or for any other related obligations of the company.
The Articles also permit us to purchase insurance on behalf of any officer, director, employee or other agent of our company, of an affiliated entity, or, at our request, of another entity, for any liability arising out of that person’s actions in such capacity. We have entered into indemnification agreements with each of our current directors and executive officers requiring us to indemnify these individuals to the fullest extent permitted under British Columbia law against liability that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified, and have received a written undertaking from each such director and officer as required under British Columbia law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Shareholder Rights Plan
We adopted a shareholder rights plan agreement (Rights Plan). The Rights Plan is designed to provide adequate time for the Board of Directors and the shareholders to assess an unsolicited takeover bid for DiaMedica, to provide the Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The Rights Plan was renewed at the Company’s annual general meeting of shareholders in December 2017 and is set to expire at the close of the Company’s annual general and special meeting of shareholders in 2020.
The rights issued under the Rights Plan will initially attach to and trade with the common shares, and no separate certificates will be issued unless an event triggering these rights occurs. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of the outstanding common shares without complying with the “Permitted Bid” provisions of the Rights Plan or without approval of the Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time.
Under the Rights Plan, a Permitted Bid is a bid made to all holders of the common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
The issuance of common shares upon the exercise of the rights is subject to receipt of certain regulatory approvals.
Anti-takeover Laws
In Canada, takeover bids are governed by provincial corporate and securities laws and the rules of applicable stock exchanges. The following description of the rules relating to acquisitions of securities and takeover bids to which Canadian corporate and securities laws apply does not purport to be complete and is subject, and qualified in its entirety by reference, to applicable corporate and securities laws, which may vary from province to province.
A party (acquirer) who acquires beneficial ownership of, or control or direction over, more than 10% of the voting or equity securities of any class of a reporting issuer (or securities convertible into voting or equity securities of any class of a reporting issuer) will generally be required to file with applicable provincial regulatory authorities both a news release and a report containing the information prescribed by applicable securities laws. Subject to the below, the acquirer (including any party acting jointly or in concert with the acquirer) will be prohibited from purchasing any additional securities of the class of the target company previously acquired for a period commencing on the occurrence of an event triggering the aforementioned filing requirement and ending on the expiry of one business day following the filing of the report. This filing process and the associated restriction on further purchases also apply in respect of subsequent acquisitions of 2% or more of the securities of the same class (or securities convertible into voting or equity securities of any class of a reporting issuer). The restriction on further purchases does not apply to an acquirer that beneficially owns, or controls or directs, 20% or more of the outstanding securities of that class.
In addition to the foregoing, certain other Canadian legislation may limit a Canadian or non-Canadian entity’s ability to acquire control over or a significant interest in us, including the Competition Act (Canada) and the Investment Canada Act (Canada). Issuers may also approve and adopt shareholder rights plans or other defensive tactics designed to be triggered upon the commencement of an unsolicited bid and make the company a less desirable takeover target.
Other Canadian Laws Affecting U.S. Shareholders
There are no governmental laws, decrees or regulations in Canada relating to restrictions on the export or import of capital, or affecting the remittance of interest, dividends or other payments by us to non-residents of Canada.
Dividends paid or credited (or deemed to be paid or credited) by the Company to residents of the United States of America within the meaning of the Canada-United States Tax Convention (1980), as amended (US Treaty) are generally subject to a 15% withholding tax on the gross amount of the dividends (see discussion below under “Material Canadian Federal Income Tax Considerations—Dividends”).
There are no limitations specific to the rights of non-residents of Canada to hold or vote our common shares under the BCBCA, or in our notice of articles or articles, other than those imposed by the Investment Canada Act (Canada) as discussed below.
Non-Canadian investors who acquire a controlling interest in us may be subject to the Investment Canada Act (Canada), which governs the basis on which non-Canadians may invest in Canadian businesses. Under the Investment Canada Act (Canada), the acquisition of a majority of the voting interests of an entity (or of a majority of the undivided ownership interests in the voting common shares of an entity that is a corporation) is deemed to be an acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of the voting common shares. The acquisition of less than one-third of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is deemed not to be acquisition of control of that corporation.
Differences in Corporate Law
We are governed by the BCBCA, which is generally similar to laws applicable to United States corporations. Significant differences between the BCBCA and the Delaware General Corporate Law (DGCL), which governs companies incorporated in the State of Delaware, include the following:
|
Capital Structure |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the certificate of incorporation must set forth the total number of shares of stock which the corporation shall have authority to issue and the par value of each of such shares, or a statement that the shares are to be without par value. |
Under the BCBCA, the notice of articles of a corporation must describe the authorized share structure of the corporation. |
|
Dividends |
||
|
Delaware |
British Columbia |
|
|
The DGCL generally provides that, subject to certain restrictions, the directors of a corporation may declare and pay dividends upon the shares of its capital stock either out of the corporation’s surplus or, if there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. Further, the holders of preferred or special stock of any class or series may be entitled to receive dividends at such rates, on such conditions and at such times as stated in the certificate of incorporation. |
Under the BCBCA, dividends may be declared on the common shares at the discretion of the board of directors. Any dividends declared shall be subject to the rights, if any, of shareholders holding shares with special rights as to dividends.
Our directors may declare dividends unless there are reasonable grounds for believing that the corporation is insolvent or the payment of such dividends would render the company insolvent. |
|
|
Number and Election of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the board of directors must consist of at least one person, and the number of directors is generally fixed by, or in the manner provided in, the bylaws of the corporation, unless the certificate of incorporation fixes the number of directors, in which case a change in the number of directors shall be made only by amendment of the certificate.
The Board may be divided into three classes of directors, with one-third of each class subject to election by the stockholder each year after such classification becomes effective. |
Pursuant to the BCBCA, a public company must have at least three directors.
In accordance with our Articles, all directors cease to hold office immediately before the election or appointment of directors at every annual general meeting of shareholders, but are eligible for re-election or re-appointment. |
|
|
Removal of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, any or all directors may be removed with or without cause by the holders of a majority of shares entitled to vote at an election of directors unless the certificate of incorporation otherwise provides or in certain other circumstances if the corporation has cumulative voting. |
As permitted under the BCBCA, our Articles provide that a director may be removed before the expiration of their term by a special resolution of shareholders. Our Articles also provide that the directors may remove any director before the expiration of their term if the director is charged with an indictable offence or if the director ceases to be qualified to act as a director and does not promptly resign, and the directors may appoint a director to fill the resulting vacancy. |
|
|
Vacancies on the Board of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, vacancies and newly created directorships resulting from an increase in the authorized number of directors may be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director. |
Under the BCBCA, casual vacancies on the board may be filled by the remaining directors. If a vacancy on the board occurs as a result of the removal of a director, the vacancy may be filled by the shareholders at the shareholders meeting, if any, at which the director is removed, or if not filled in that manner, by the shareholders or the remaining directors. |
|
Qualifications of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, directors are required to be natural persons, but are not required to be residents of Delaware. The certificate of incorporation or bylaws may prescribe other qualifications for directors. |
Under the BCBCA, directors are not required to be residents of British Columbia. The articles of a corporation may prescribe other qualifications for directors. |
|
|
Board of Director Quorum and Vote Requirements |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a majority of the total number of directors shall constitute a quorum for the transaction of business unless the certificate or bylaws require a greater number. The bylaws may lower the number required for a quorum to one-third the number of directors, but no less.
Under the DGCL, the board of directors may take action by the majority vote of the directors present at a meeting at which a quorum is present unless the certificate of incorporation or bylaws require a greater vote. |
Under the BCBCA, a majority of the number of directors or minimum number of directors required by the articles constitutes a quorum at any meeting. |
|
|
Transactions with Directors and Officers |
||
|
Delaware |
British Columbia |
|
|
The DGCL generally provides that no transaction between a corporation and one or more of its directors or officers, or between a corporation and any other corporation or other organization in which one or more of its directors or officers, are directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the board or committee which authorizes the transaction, or solely because any such director’s or officer’s votes are counted for such purpose, if: (i) the material facts as to the director’s or officer’s interest and as to the transaction are known to the board of directors or the committee, and the board or committee in good faith authorizes the transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; (ii) the material facts as to the director’s or officer’s interest and as to the transaction are disclosed or are known to the stockholders entitled to vote thereon, and the transaction is specifically approved in good faith by vote of the stockholders; or (iii) the transaction is fair as to the corporation as of the time it is authorized, approved or ratified, by the board of directors, a committee or the stockholders. |
Under the BCBCA, a director or senior officer who holds a disclosable interest in a material contract or transaction into which a corporation has entered or proposes to enter may generally not vote on any directors’ resolution to approve the contract or transaction. A director or senior officer has a disclosable interest in a material contract or transaction if (a) the contract or transaction is material to the corporation, (b) the corporation has entered, or proposes to enter, into the contract or transaction, and (c) either of the following applies to the director or senior officer: (i) the director or senior officer has a material interest in the contract or transaction, or (ii) the director or senior officer is a director or senior officer of, or has a material interest in, a person who has a material interest in the contract or transaction.
Under the BCBCA, directors or senior officers do not have a disclosable interest in a contract or transaction merely because the contract or transaction relates to the remuneration of the director or senior officer in that person’s capacity as director, officer, employee or agent of the corporation or of an affiliate of the corporation. |
|
|
Limitation on Liability of Directors |
||
|
Delaware |
British Columbia |
|
|
The DGCL permits a corporation to include a provision in its certificate of incorporation eliminating or limiting the personal liability of a director to the corporation or its stockholders for monetary damages for a breach of the director’s fiduciary duty as a director, except: ● for breach of the director’s duty of loyalty to the corporation or its stockholders; ● for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of the law; ● under Section 174 of the DGCL, which concerns unlawful payment of dividends, stock purchases or redemptions; or ● for any transaction from which the director derived an improper personal benefit. |
No provision in a contract or the articles relieves a director or officer from the duty to act in accordance with the BCBCA and the regulations, or relieves them from liability for a breach thereof. |
|
Indemnification of Directors and Officers |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a corporation may indemnify any person who is made a party to any third-party action, suit or proceeding on account of being a director, officer, employee or agent of the corporation (or was serving at the request of the corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise) against expenses, including attorney’s fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with the action, suit or proceeding through, among other things, a majority vote of a quorum consisting of directors who were not parties to the suit or proceeding, if the person: ● acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation; ● in some circumstances, acted at least not opposed to its best interests; and ● in a criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL permits indemnification for derivative suits against expenses (including legal fees) if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and only if the person is not found liable, unless a court determines the person is fairly and reasonably entitled to the indemnification. |
Under the BCBCA, a corporation may indemnify a director or officer of the corporation, a former director or officer of the corporation, or another individual who acts or acted at the corporation’s request as a director or officer, or an individual acting in a similar capacity, of another entity (an “eligible party”), against all judgments, penalties or fines awarded or imposed in, or an amount paid in settlement of (an “eligible penalty”) a proceeding in which the eligible party or any of the heirs and personal representatives of the eligible party, by reason of the eligible party being or having been a director or officer of, or holding or having held a position equivalent to that of a director or officer, the corporation or an associated corporation is or may be joined as a party, or is or may be liable for or in respect of a judgment, penalty or fine in, or expenses related to, the proceeding (an “eligible proceeding”).
Under the BCBCA, a corporation must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by the eligible party in respect of that proceeding if the eligible party has not been reimbursed for those expenses and is wholly successful, on the merits or otherwise, in the outcome of the proceeding or is substantially successful on the merits in the outcome of the proceeding.
Under the BCBCA, a corporation may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an eligible party in respect of that proceeding. Notwithstanding the foregoing, a corporation must not make any such payments unless the corporation first receives from the eligible party a written undertaking that, if it is ultimately determined that the payment of the expenses is prohibited under the BCBCA, the eligible party will repay the amounts advanced. |
|
A corporation may not indemnity an eligible party or pay the expenses of an eligible party: ● if, in relation to the subject matter of the eligible proceeding, the eligible party did not act honestly and in good faith with a view to the best interests of the corporation or the associated corporation, as the case may be; ● in the case of an eligible proceeding other than a civil proceeding, if the eligible party did not have reasonable grounds for believing that the eligible party’s conduct in respect of which the proceeding was brought was lawful.
If an eligible proceeding is brought against an eligible party by or on behalf of the corporation or by or on behalf of an associated corporation, the corporation must not indemnify an eligible party in respect of the proceeding or pay the expenses of the eligible party in respect of the proceeding. |
||
|
Call and Notice of Shareholder Meetings |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, an annual or special stockholder meeting is held on such date, at such time and at such place as may be designated by the board of directors or any other person authorized to call such meeting under the corporation’s certificate of incorporation or bylaws.
If an annual meeting for election of directors is not held on the date designated or an action by written consent to elect directors in lieu of an annual meeting has not been taken within 30 days after the date designated for the annual meeting, or if no date has been designated, for a period of 13 months after the later of the last annual meeting or the last action by written consent to elect directors in lieu of an annual meeting, the Delaware Court of Chancery may summarily order a meeting to be held upon the application of any stockholder or director.
Special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
Under the BCBCA, the directors are required to call an annual meeting of shareholders not later than 18 months after the date the corporation was recognized, and subsequently, at least once in each calendar year and not more than 15 months after the last annual reference date.
As permitted by the BCBCA, our Articles stipulate that a meeting of our shareholders may be held in our outside of British Columbia as determined by the board of directors.
The directors may at any time call a special meeting of the shareholders. The holders of not less than five per cent of the issued shares of a corporation that carry the right to vote at a meeting may requisition the directors to call a meeting of shareholders for the purposes stated in the requisition. |
|
|
Shareholder Action by Written Consent |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a majority of the stockholders of a corporation may act by written consent without a meeting unless such action is prohibited by the corporation’s certificate of incorporation. |
Under the BCBCA, shareholders may act by written resolution signed by all the shareholders entitled to vote on that resolution at a meeting of shareholders. |
|
Shareholder Nominations and Proposals |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the bylaws of a corporation may include provisions respecting the nomination of directors or proposals by stockholders, including requirements for advance notice to the corporation. |
Subject to the BCBCA, a registered owner or beneficial owner of one or more shares that carry the right to vote at general meetings and who has been a registered owner or beneficial owner of one or more such shares for an uninterrupted period of at least 2 years may submit to the corporation notice of any matter that the person wishes to have considered at the next annual general meeting of the corporation. |
|
|
Shareholder Quorum and Vote Requirements |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, quorum for a stock corporation is a majority of the shares entitled to vote at the meeting unless the certificate of incorporation or bylaws specify a different quorum, but in no event may a quorum be less than one-third of the shares entitled to vote. Unless the DGCL, certificate of incorporation or bylaws provide for a greater vote, generally the required vote under the DGCL is a majority of the shares present in person or represented by proxy, except for the election of directors which requires a plurality of the votes cast. |
Unless the articles otherwise provide, under the BCBCA a quorum of shareholders is present at a meeting of shareholders, irrespective of the number of persons actually present at the meeting, if the holders of a majority of the shares entitled to vote at the meeting are present in person or represented by proxy. Under our articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding, in the aggregate, not less than 33 1/3% of the outstanding voting common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting.
Unless the BCBCA or articles provide for a greater vote, generally the required vote under the BCBCA is by ordinary resolution, or a resolution passed by a majority of the votes cast by the shareholders who voted in respect of that resolution. |
|
|
Amendment of Governing Instrument |
||
|
Delaware |
British Columbia |
|
|
Amendment of Certificate of Incorporation. Generally, under the DGCL, the affirmative vote of the holders of a majority of the outstanding stock entitled to vote is required to approve a proposed amendment to the certificate of incorporation, following the adoption of the amendment by the board of directors of the corporation, provided that the certificate of incorporation may provide for a greater vote. Under the DGCL, holders of outstanding shares of a class or series are entitled to vote separately on an amendment to the certificate of incorporation if the amendment would have certain consequences, including changes that adversely affect the rights and preferences of such class or series.
Amendment of Bylaws. Under the DGCL, after a corporation has received any payment for any of its stock, the power to adopt, amend or repeal bylaws shall be vested in the stockholders entitled to vote; provided, however, that any corporation may, in its certificate of incorporation, provide that bylaws may be adopted, amended or repealed by the board of directors. The fact that such power has been conferred upon the board of directors shall not divest the stockholders of the power nor limit their power to adopt, amend or repeal the bylaws. |
Amendment to Notice of Articles. Under the BCBCA, an amendment to a corporation’s notice of articles generally requires a special resolution of shareholders. A special resolution is a resolution passed by a majority of not less than two-thirds of the votes cast by the shareholders who voted in respect of the resolution or signed by all shareholders entitled to vote on that resolution.
Amendment of Articles. Unless the articles otherwise provide, the directors may, by resolution, make, amend or repeal any articles that regulate the business or affairs of the corporation. |
|
Votes on Mergers, Consolidations and Sales of Assets |
||
|
Delaware |
British Columbia |
|
|
The DGCL provides that, unless otherwise provided in the certificate of incorporation or bylaws, the adoption of a merger agreement requires the approval of a majority of the outstanding stock of the corporation entitled to vote thereon. |
Under the BCBCA, the approval of an amalgamation agreement requires approval by special resolution. |
|
|
Dissenters’ Rights of Appraisal |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a stockholder of a Delaware corporation generally has the right to dissent from and request payment for the stockholders shares upon a merger or consolidation in which the Delaware corporation is participating, subject to specified procedural requirements, including that such dissenting stockholder does not vote in favor of the merger or consolidation. However, the DGCL does not confer appraisal rights, in certain circumstances, including if the dissenting stockholder owns shares traded on a national securities exchange and will receive publicly traded shares in the merger or consolidation. Under the DGCL, a stockholder asserting appraisal rights does not receive any payment for his or her shares until the court determines the fair value or the parties otherwise agree to a value. The costs of the proceeding may be determined by the court and assessed against the parties as the court deems equitable under the circumstances. |
Under the BCBCA, a shareholder may dissent from a transaction when the corporation resolves to: (a) amend its articles to alter a restriction on the powers of the corporation or on the business the corporation is permitted to carry on; (b) adopt an amalgamation agreement; (c) to approve an arrangement, the terms of which arrangement permit dissent; (d) authorize or ratify the sale, lease or other disposition of all or substantially all of the corporation’s undertaking; (e) be continued under the laws of another jurisdiction.
A shareholder asserting dissenters rights is entitled, subject to specified procedural requirements, including objecting to the action giving rise to dissenters rights and making a proper demand for payment, to be paid by the corporation the fair value of the shares in respect of which the shareholder dissents. Under the BCBCA, if the shareholder and the corporation do not agree on the fair value for the shareholders shares, the corporation or the dissenting shareholder may apply to a court to fix a fair value for the shares. |
|
|
Anti-takeover and Ownership Provisions |
||
|
Delaware |
British Columbia |
|
|
Unless an issuer opts out of the provisions of Section 203 of the DGCL, Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with a holder of 15% or more of the corporation’s voting stock (as defined in Section 203), referred to as an interested stockholder, for a period of three years after the date of the transaction in which the interested stockholder became an interested stockholder, except as otherwise provided in Section 203. For these purposes, the term “business combination” includes mergers, assets sales and other similar transactions with an interested stockholder. |
The BCBCA contains no restriction on adoption of a shareholder rights plan. The BCBCA does not restrict related party transactions; however, in Canada, takeovers and other related party transactions are addressed in provincial securities legislation and policies. |
CERTAIN UNITED STATES INCOME TAX CONSIDERATIONS
The following discussion is generally limited to certain material U.S. federal income tax considerations relating to the purchase, ownership and disposition of our common shares by U.S. Holders (as defined below). This discussion applies to U.S. Holders that hold our common shares as capital assets. This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of our common shares. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Although this discussion is generally limited to the U.S. federal income tax considerations to U.S. Holders, the U.S. federal income tax treatment of dividends on and gain on sale or exchange of our common shares by certain “Non-U.S. Holders” (as defined below) is included below at “U.S. Federal Income Taxation of Non-U.S. Holders.”
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (IRS) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions presented in this summary. In addition, because the guidance on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions described in this summary.
This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (Code), U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, and the income tax treaty between the United States and Canada (Convention), all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect. This summary is applicable to U.S. Holders who are residents of the United States for purposes of the Convention and who qualify for the full benefits of the Convention. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
This discussion does not address all of the U.S. federal income tax considerations that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents of the United States, persons who hold common shares as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons that have a “functional currency” other than the U.S. dollar, persons that own (or are deemed to own) 10% or more (by voting power or value) of our common shares, persons that acquire their common shares as part of a compensation arrangement, corporations that accumulate earnings to avoid U.S. federal income tax, partnerships and other pass-through entities, and investors in such pass-through entities). This discussion does not address any U.S. state or local or non-U.S. tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements.
As used in this discussion, the term “U.S. Holder” means a beneficial owner of common shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
If an entity treated as a partnership for U.S. federal income tax purposes holds the common shares, the U.S. federal income tax considerations relating to an investment in the common shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor regarding the U.S. federal income tax considerations applicable to it and its partners of the purchase, ownership and disposition of the common shares.
Persons holding common shares should consult their own tax advisors as to the particular tax considerations applicable to them relating to the purchase, ownership and disposition of common shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Distributions
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” a U.S. Holder that receives a distribution with respect to the common shares generally will be required to include the gross amount of such distribution (before reduction for any Canadian withholding taxes) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s common shares. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s common shares, the remainder will be taxed as capital gain. However, we cannot provide any assurance that we will maintain or provide earnings and profits determinations in accordance with U.S. federal income tax principles. Therefore, U.S. Holders should expect that a distribution will generally be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
The U.S. dollar value of any distribution on the common shares made in Canadian dollars generally should be calculated by reference to the exchange rate between the U.S. dollar and the Canadian dollar in effect on the date of receipt (or deemed receipt) of such distribution by the U.S. Holder regardless of whether the Canadian dollars so received are in fact converted into U.S. dollars at that time. If the Canadian dollars received are converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally should not recognize currency gain or loss on such conversion. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally will have a basis in such Canadian dollars equal to the U.S. dollar value of such Canadian dollars on the date of receipt (or deemed receipt). Any gain or loss on a subsequent conversion or other disposition of such Canadian dollars by such U.S. Holder generally will be treated as ordinary income or loss and generally will be income or loss from sources within the United States for U.S. foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Distributions on the common shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute “passive category income.” Because we are not a United States corporation, such dividends will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations. Dividends paid by a “qualified foreign corporation” to a U.S. Holder who is an individual, trust or estate will generally be treated as “qualified dividend income” and are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are met. However, if we are a passive foreign investment company (PFIC) for the taxable year in which the dividend is paid or the preceding taxable year (see discussion below under “Passive Foreign Investment Company Considerations”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its own tax advisors regarding the availability of the reduced tax rate on dividends.
If a U.S. Holder is subject to Canadian withholding tax on dividends paid on the holder’s common shares (see discussion below under “Material Canadian Federal Income Tax Considerations—Dividends”), the U.S. Holder may be eligible, subject to a number of complex limitations, to claim a credit against its U.S. federal income tax for the Canadian withholding tax imposed on the dividends. However, if U.S. persons collectively own, directly or indirectly, 50% or more of the voting power or value of our common shares it is possible that a portion of any dividends we pay will be considered U.S. source income in proportion to our U.S. source earnings and profits, which could limit the ability of a U.S. Holder to claim a foreign tax credit for the Canadian withholding taxes imposed in respect of such a dividend, although certain elections may be available under the Code and the Convention to mitigate these effects. A U.S. Holder may claim a deduction for the Canadian withholding tax in lieu of a credit, but only for a year in which the U.S. Holder elects to do so for all creditable foreign income taxes. The rules governing the foreign tax credit are complex. Each U.S. Holder is advised to consult its tax advisor regarding the availability of the foreign tax credit under its particular circumstances.
Sale, Exchange or Other Disposition of Common Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of common shares. The amount of gain recognized will equal the excess of the amount realized (i.e., the amount of cash plus the fair market value of any property received) over the U.S. Holder’s adjusted tax basis in the common shares sold or exchanged. The amount of loss recognized will equal the excess of the U.S. Holder’s adjusted tax basis in the common shares sold or exchanged over the amount realized. Such capital gain or loss generally will be long-term capital gain or loss if, on the date of sale, exchange or other disposition, the common shares were held by the U.S. Holder for more than one year. Net long-term capital gain derived by a non-corporate U.S. Holder with respect to capital assets is currently is subject to tax at reduced rates. The deductibility of a capital loss is subject to limitations. Any gain or loss recognized from the sale, exchange or other disposition of common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes, except as otherwise provided in an applicable income tax treaty and if an election is properly made under the Code.
Passive Foreign Investment Company Considerations
In general, a corporation organized outside the United States will be treated as a PFIC in any taxable year in which either (1) at least 75% of its gross income is “passive income” or (2) at least 50% of the average quarterly value of its assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from commodities transactions and from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. The average percentage of a corporation’s assets that produce or are held for the production of passive income generally is determined on the basis of the fair market value of the corporation’s assets at the end of each quarter (which may be determined in part by the market value of our common shares, which is subject to change). In determining whether a foreign corporation is a PFIC, a proportionate share of the items of gross income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) are taken into account.
Although the tests for determining PFIC status are applied as of the end of each taxable year and are dependent upon a number of factors, some of which are beyond our control, including the value of our assets, the market price of our common shares, and the amount and type of our gross income (i) we believe that we were a PFIC for the taxable year ended December 31, 2016, and (ii) we do not believe that we were a PFIC for the taxable years ended December 31, 2019, 2018 and 2017. Our status as a PFIC is a fact-intensive determination made on an annual basis, and we cannot provide any assurance regarding our PFIC status for the taxable year ending December 31, 2020 or for subsequent taxable years. U.S. Holders who own our common shares for any period during which we are a PFIC will be required to file IRS Form 8621 for each tax year during which they hold our common shares. No opinion of legal counsel or ruling from the IRS concerning our status as a PFIC has been obtained or is currently planned to be requested. However, the determination of our PFIC status is made annually after the close of each taxable year and it is difficult to predict before such determination whether we will be a PFIC for any given taxable year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that the IRS will agree with our conclusion. No assurance can be provided regarding our PFIC status, and neither we nor our United States counsel expresses any opinion with respect to our PFIC status.
If we are a PFIC at any time when a non-corporate U.S. Holder owns common shares, and such U.S. Holder does not make a “qualified electing fund” election (“QEF election”) or a “mark-to-market” election, both as described below, such U.S. Holder will generally be subject to federal tax under the excess distribution rules (described below). Under such rules, additional taxes and interest charges would apply to certain distributions by us or to gain upon dispositions of our common shares. If neither of such elections are made, the excess distribution rules apply to (1) distributions paid during a taxable year that are greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the common shares, and (2) any gain recognized on a sale, exchange or other disposition (which would include a pledge or transfer by gift or death) of common shares. Under the excess distribution rules, the non-corporate U.S. Holder’s tax liability will be determined by allocating such distribution or gain ratably to each day in the U.S. Holder’s holding period for the common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we were a PFIC in the holding period will be taxed as ordinary income earned in the current taxable year and the preferential tax rate applicable to capital gains or dividends received on our common shares would not be available. The amount allocated to other taxable years (i.e., prior years in which we were a PFIC) will be taxed at the highest marginal rate in effect (for individuals or corporations as applicable) for ordinary income in each such taxable year, and an interest charge, generally applicable to the underpayment of tax, will be added to the tax and the preferential tax rate applicable to capital gains or dividends received on our common shares would not be available. These adverse tax consequences would not apply to a pension or profit-sharing trust or other tax-exempt organization that did not borrow funds or otherwise utilize leverage in connection with its acquisition of our common shares. In addition, if a non-electing U.S. Holder who is an individual dies while owning our common shares, such U.S. Holder's successor generally would not receive a step-up in tax basis with respect to such common shares, but instead would have a tax basis equal to the lower of the fair market value of such common shares or the decedent’s tax basis in such common shares.
If we are a PFIC at any time when a U.S. Holder holds our common shares, we will generally continue to be treated as a PFIC with respect to the U.S. Holder for all succeeding years during which the U.S. Holder holds our common shares even if we cease to meet the PFIC gross income test or asset test in a subsequent year. However, if we cease to meet these tests, a U.S. Holder can avoid the continuing impact of the PFIC rules by making a special election (a “Purging Election”) to recognize gain by making a “deemed sale” election with respect to all of the U.S. Holder’s common shares and have such common shares deemed to be sold at their fair market value on the last day of the last taxable year during which we were a PFIC. In addition, for a U.S. Holder making such an election, a new holding period would be deemed to begin for our common shares for purposes of the PFIC rules. After the Purging Election, the common shares with respect to which the Purging Election was made will not be treated as shares in a PFIC unless we subsequently again become a PFIC.
The tax considerations that would apply if we were a PFIC would be different from those described above if a U.S. Holder were able to make a valid QEF election. For each year that we meet the PFIC gross income test or asset test, an electing U.S. Holder would be required to include in gross income its pro rata share of our ordinary income and net capital gains, if any, as determined under U.S. federal income tax principles. The U.S. Holder’s adjusted tax basis in our common shares would be increased by the amount of such inclusions. An actual distribution to the U.S. Holder out of such income generally would not be treated as a dividend and would decrease the U.S. Holder’s adjusted tax basis in our common shares. Gain realized from the sale of our common shares covered by a QEF election would be taxed as a capital gain and the denial of the basis step-up at death described above would not apply. Generally, a QEF election must be made by the U.S. Holder in a timely filed tax return for the first taxable year in which the U.S. Holder held our common shares that includes the close of our taxable year for which we met the PFIC gross income test or asset test. A separate QEF election would need to be made for any of our subsidiaries that are classified as a PFIC. A QEF election is made on IRS Form 8621. U.S. Holders will be eligible to make QEF elections only if we agree to provide U.S. Holders with the information they will need to comply with the QEF rules. In the event we become a PFIC, we intend to provide all information and documentation that a U.S. Holder making a QEF election is required to obtain for U.S. federal income tax purposes (e.g., the U.S. Holder’s pro rata share of ordinary income and net capital gain, and a “PFIC Annual Information Statement” as described in applicable U.S. Treasury regulations).
As an alternative to a QEF election, a U.S. Holder may also mitigate the adverse tax consequences of PFIC status by timely making a “mark-to-market” election, provided the U.S. Holder completes and files IRS Form 8621 in accordance with the relevant instructions and related Treasury regulations. A U.S. Holder who makes the mark-to-market election generally must include as ordinary income each year the increase in the fair market value of the common shares and deduct from gross income the decrease in the value of such shares during each of its taxable years, but with losses limited to the amount of previously recognized net gains. The U.S. Holder’s tax basis in the common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. If a mark-to-market election with respect to our common shares is in effect on the date of a U.S. Holder’s death, the tax basis of the common shares in the hands of a U.S. Holder who acquired them from a decedent will be the lesser of the decedent’s tax basis or the fair market value of the common shares. Any gain from a sale, exchange or other disposition of the common shares in any taxable year in which we are a PFIC (i.e., when we meet the gross income test or asset test described above) would be treated as ordinary income and any loss from a sale, exchange or other disposition would be treated first as an ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as a capital loss. If we cease to be a PFIC, any gain or loss recognized by a U.S. Holder on the sale or exchange of the common shares would be classified as a capital gain or loss.
A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable U.S. Treasury regulations. A class of stock is regularly traded during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. The common shares should be marketable stock as long as they are listed on The Nasdaq Capital Market and are regularly traded. A mark-to-market election will not apply to the common shares for any taxable year during which we are not a PFIC but will remain in effect with respect to any subsequent taxable year in which we again become a PFIC. Such election will not apply to any subsidiary that we own. Accordingly, a U.S. Holder may continue to be subject to the PFIC rules with respect to any lower-tier PFICs notwithstanding the U.S. Holder’s mark-to-market election. Whether our common shares are regularly traded on a qualified exchange is an annual determination based on facts that, in part, are beyond our control. Accordingly, a U.S. Holder might not be eligible to make a mark-to-market election to mitigate the adverse tax consequences if we are characterized as a PFIC.
Each U.S. person who is a shareholder of a PFIC generally must file an annual report (on IRS Form 8621) with the IRS containing certain information, and the failure to file such report could result in the imposition of penalties on such U.S. person and in the extension of the statute of limitations with respect to federal income tax returns filed by such U.S. person.
The U.S. federal income tax rules relating to PFICs are very complex. U.S. Holders are urged to consult their own tax advisors with respect to the purchase, ownership and disposition of common shares, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of common shares in the event we are considered a PFIC.
Additional Tax on Passive Income
Certain U.S. Holders that are individuals, estates or trusts (other than trusts that are exempt from tax) with adjusted income exceeding certain thresholds, will be subject to a 3.8% tax on all or a portion of their “net investment income,” which includes dividends on the common shares, and net gains from the disposition of the common shares. Further, excess distributions treated as dividends, gains treated as excess distributions, and mark-to-market inclusions and deductions are all included in the calculation of net investment income.
Treasury regulations provide, subject to the election described in the following paragraph, that solely for purposes of this additional tax, that distributions of previously taxed income will be treated as dividends and included in net investment income subject to the additional 3.8% tax. Additionally, to determine the amount of any capital gain from the sale or other taxable disposition of common shares that will be subject to the additional tax on net investment income, a U.S. Holder who has made a QEF election will be required to recalculate its basis in the common shares excluding any QEF election basis adjustments.
Alternatively, a U.S. Holder may make an election which will be effective with respect to all interests in controlled foreign corporations and PFICs that are subject to a QEF election and that are held in that year or acquired in future years. Under this election, a U.S. Holder pays the additional 3.8% tax on QEF election income inclusions and on gains calculated after giving effect to related tax basis adjustments. U.S. Holders that are individuals, estates or trusts should consult their own tax advisors regarding the applicability of this tax to any of their income or gains in respect of the common shares.
U.S. Federal Income Taxation of Non-U.S. Holders
A beneficial owner of our common shares, other than a partnership or entity treated as a partnership for U.S. Federal income tax purposes, that is not a U.S. Holder is referred to herein as a “Non-U.S. Holder”. Non-U.S. Holders generally will not be subject to U.S. federal income tax or withholding tax on dividends received from us with respect to our common shares, unless that income is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States. In general, if the Non-U.S. Holder is entitled to the benefits of certain U.S. income tax treaties with respect to those dividends, that income is taxable only if it is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States.
Non-U.S. Holders generally will not be subject to U.S. federal income tax or withholding tax on any gain realized upon the sale, exchange or other disposition of our common shares, unless:
|
● |
the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States. In general, if the Non-U.S. Holder is entitled to the benefits of certain income tax treaties with respect to that gain, that gain is taxable only if it is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States; or |
|
● |
the Non-U.S. Holder is an individual who is present in the United States for 183 days or more during the taxable year of disposition and other conditions are met. |
If the Non-U.S. Holder is engaged in a U.S. trade or business for U.S. federal income tax purposes, the income from the common shares, including dividends and the gain from the sale, exchange or other disposition of the stock, that is effectively connected with the conduct of that trade or business will generally be subject to regular U.S. federal income tax in the same manner as discussed above relating to the general taxation of U.S. Holders. In addition, if you are a corporate Non-U.S. Holder, your earnings and profits that are attributable to the effectively connected income, which are subject to certain adjustments, may be subject to an additional branch profits tax at a rate of 30%, or at a lower rate as may be specified by an applicable U.S. income tax treaty.
Information Reporting with Respect to Foreign Financial Assets
U.S. individuals that own “specified foreign financial assets” (as defined in Section 6038D of the Code) with an aggregate fair market value exceeding certain threshold amounts generally are required to file an information report on IRS Form 8938 with respect to such assets with their tax returns. Significant penalties may apply to persons who fail to comply with these rules. Specified foreign financial assets include not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by certain financial institutions, any stock or security issued by a non-U.S. person, such as our common shares. Upon the issuance of future U.S. Treasury regulations, these information reporting requirements may apply to certain U.S. entities that own specified foreign financial assets. The failure to report information required under the current regulations could result in substantial penalties and in the extension of the statute of limitations with respect to federal income tax returns filed by a U.S. Holder. U.S. Holders should consult their own tax advisors regarding the possible implications of these U.S. Treasury regulations for an investment in our common shares.
Special Reporting Requirements for Transfers to Foreign Corporations
A U.S. Holder that acquires common shares generally will be required to file IRS Form 926 with the IRS if (1) immediately after the acquisition such U.S. Holder, directly or indirectly, owns at least 10% of our common shares, or (2) the amount of cash transferred in exchange for common shares during the 12-month period ending on the date of the acquisition exceeds USD $100,000. Significant penalties may apply for failing to satisfy these filing requirements. U.S. Holders are urged to contact their tax advisors regarding these filing requirements.
Information Reporting and Backup Withholding
Dividends on and proceeds from the sale or other disposition of common shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if (1) the U.S. holder fails to provide an accurate taxpayer identification number or otherwise establish a basis for exemption, (2) the U.S. Holder is notified by the IRS that backup withholding applies, or (3) the payment is described in certain other categories of persons.
If you sell your common shares through a U.S. office of a broker, the payment of the proceeds is subject to both U.S. backup withholding and information reporting unless you certify that you are a non-U.S. person, under penalties of perjury, or you otherwise establish an exemption. If you sell your common shares through a non-U.S. office of a non-U.S. broker and the sales proceeds are paid to you outside the United States, then information reporting and backup withholding generally will not apply to that payment. However, U.S. information reporting requirements, but not backup withholding, will apply to a payment of sales proceeds, even if that payment is made to you outside the United States, if you sell your common shares through a non-U.S. office of a broker that is a U.S. person or has certain other contacts with the United States, unless you certify that you are a non-U.S. person, under penalty of perjury, or you otherwise establish an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A U.S. HOLDER. EACH U.S. HOLDER IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of February 10, 2020, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (Tax Act) generally applicable to a holder of our common shares who, for purposes of the Tax Act and at all relevant times, is neither resident in Canada nor deemed to be resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention, and who does not use or hold (and is not deemed to use or hold) common shares in the course of carrying on a business in Canada, deals at arm’s length with and is not affiliated with us and holds our common shares as capital property (Holder). Generally, common shares will be considered to be capital property to a Holder thereof provided that the Holder does not hold common shares in the course of carrying on a business and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder, (i) that is a “financial institution” for purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii) that holds an interest which is a “tax shelter investment” as defined in the Tax Act; or (iv) that has elected to report its tax results in a functional currency other than Canadian currency. Special rules, which are not discussed in this summary, may apply to a Holder that is an “authorized foreign bank” within the meaning of the Tax Act, a partnership or an insurer carrying on business in Canada and elsewhere. Such Holders should consult their own tax advisors.
This summary is based upon the provisions of the Tax Act (including the regulations (Regulations) thereunder) in force as of February 10, 2020 and our understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (CRA) published in writing by the CRA prior to February 10, 2020. This summary takes into account all specific proposals to amend the Tax Act (and the Regulations) publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (Tax Proposals) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action. This summary is not exhaustive of all possible Canadian federal income tax considerations and does not take into account other federal or any provincial, territorial or foreign income tax legislation or considerations, which may differ materially from those described in this summary.
This summary is of a general nature only and is not, and is not intended to be, and should not be construed to be, legal or tax advice to any particular Holder, and no representations concerning the tax consequences to any particular Holder are made. Holders should consult their own tax advisors regarding the income tax considerations applicable to them having regard to their particular circumstances.
Dividends
Dividends paid or credited (or deemed to be paid or credited) to a Holder by us are subject to Canadian withholding tax at the rate of 25% unless reduced by the terms of an applicable tax treaty or convention. For example, under the US Treaty, as amended, the dividend withholding tax rate is generally reduced to 15% in respect of a dividend paid or credited to a Holder beneficially entitled to the dividend who is resident in the United States for purposes of the US Treaty and whose entitlement to the benefits of the US Treaty is not limited by the limitation of benefits provisions of the US Treaty. Holders are urged to consult their own tax advisors to determine their entitlement to relief under the US Treaty or any other applicable tax treaty as well as their ability to claim foreign tax credits with respect to any Canadian withholding tax, based on their particular circumstances.
Disposition of Common Shares
A Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a common share, unless the common share constitutes or is deemed to constitute “taxable Canadian property” to the Holder thereof for purposes of the Tax Act, and the gain is not exempt from tax pursuant to the terms of an applicable tax treaty or convention.
In general, provided the common shares are listed on a “designated stock exchange” (which currently includes The Nasdaq Capital Market) at the date of the disposition, the common shares will only constitute “taxable Canadian property” of a Holder if, at any time within the 60-month period preceding the disposition: (i) such Holder, persons with whom the Holder did not deal at arm’s length, partnerships in which the Holder or a person with whom the Holder did not deal at arm’s length holds a membership interest directly or indirectly through one or more partnerships, or any combination thereof, owned 25% or more of the issued shares of any class or series of the Company’s share capital; and (ii) more than 50% of the fair market value of the common shares was derived directly or indirectly from one or any combination of (A) real or immovable property situated in Canada, (B) Canadian resource properties, (C) timber resource properties, and (D) options in respect of, or interests in, or for civil law rights in, property described in any of subparagraphs (ii)(A) to (C), whether or not the property exists. However, and despite the foregoing, in certain circumstances the common shares may be deemed to be “taxable Canadian property” under the Tax Act.
Holders whose common shares may be “taxable Canadian property” should consult their own tax advisers.
UNDERWRITING
We are offering the common shares described in this prospectus supplement and the accompanying prospectus through the underwriter listed below. Craig-Hallum Capital Group LLC is acting as the sole managing underwriter of this offering. The underwriter named below has agreed to buy, subject to the terms of the underwriting agreement, the number of common shares listed opposite its name below. The underwriter is committed to purchase and pay for all of the shares if any are purchased.
|
Underwriter |
|
Number of Shares |
|
Craig-Hallum Capital Group LLC |
2,125,000 |
|
|
Total |
|
2,125,000 |
The underwriter has advised us that it proposes to offer the common shares to the public at a price of $4.00 per share. The underwriter proposes to offer the common shares to certain dealers at the same price less a concession of not more than $0.28 per share. After the offering, these figures may be changed by the underwriter.
The shares sold in this offering are expected to be ready for delivery on or about February 13, 2020, against payment in immediately available funds. The underwriter may reject all or part of any order.
The table below summarizes the underwriting discounts that we will pay to the underwriter. In addition to the underwriting discount, we have agreed to pay up to $80,000 of the fees and expenses of the underwriter, which may include the fees and expenses of counsel to the underwriter. The fees and expenses of the underwriter that we have agreed to reimburse are not included in the underwriting discounts set forth in the table below. The underwriting discount and reimbursable expenses the underwriter will receive were determined through arm’s length negotiations between us and the underwriter.
|
Per Share |
Total |
|||||||
|
Underwriting discount to be paid by us |
$ | 0.28 | $ | 595,000 | ||||
We estimate that the total expenses of this offering, excluding underwriting discounts, will be approximately $205,000. This includes $80,000 of the fees and expenses of the underwriter. These expenses are payable by us.
We also have agreed to indemnify the underwriter against certain liabilities, including civil liabilities under the Securities Act of 1933, as amended, or to contribute to payments that the underwriter may be required to make in respect of those liabilities.
No Sales of Similar Securities
We, each of our directors and officers and certain of our stockholders have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any common shares or any securities convertible into or exchangeable for common shares without the prior written consent of the underwriter for a period of 90 days after the date of this prospectus supplement. These lock-up agreements provide limited exceptions, and their restrictions may be waived at any time by the underwriter.
Price Stabilization, Short Positions and Penalty Bids
To facilitate this offering, the underwriter may engage in transactions that stabilize, maintain or otherwise affect the price of our common shares during and after the offering. Specifically, the underwriter may over-allot or otherwise create a short position in our common shares for its own account by selling more common shares than we have sold to the underwriter. The underwriter may close out any short position by purchasing shares in the open market.
In addition, the underwriter may stabilize or maintain the price of our common shares by bidding for or purchasing shares in the open market and may impose penalty bids. If penalty bids are imposed, selling concessions allowed to broker-dealers participating in this offering are reclaimed if shares previously distributed in this offering are repurchased, whether in connection with stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market price of our common shares at a level above that which might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of our common shares to the extent that it discourages resales of our common shares. The magnitude or effect of any stabilization or other transactions is uncertain. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter and selling group members may also engage in passive market making transactions in our common shares on the Nasdaq Capital Market. Passive market making consists of displaying bids on the Nasdaq Capital Market limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the Securities and Exchange Commission limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of our common shares at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common shares. In addition, neither we nor the underwriter make any representation that the underwriter will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Affiliations
The underwriter and its affiliates is a full service financial institution engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. The underwriter may in the future receive customary fees and commissions for these transactions.
In the ordinary course of its various business activities, the underwriter and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for its own account and for the accounts of its customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Electronic Offer, Sale and Distribution
In connection with this offering, the underwriter or certain of the securities dealers may distribute prospectuses by electronic means, such as e-mail. In addition, the underwriter may facilitate Internet distribution for this offering to certain of its Internet subscription customers. The underwriter may allocate a limited number of shares for sale to its online brokerage customers. An electronic prospectus is available on the Internet websites maintained by any such underwriter. Other than the prospectus in electronic format, the information on the websites of the underwriter is not part of this prospectus supplement or the accompanying prospectus.
Listing
Our common shares are listed and trade in the United States on The Nasdaq Capital Market under the trading symbol “DMAC.”
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
Selling Restrictions
Canada. Our common shares may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of our common shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area. In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any of our common shares may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any of our common shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
|
● |
to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
|
● |
to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer; or |
|
● |
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of our common shares shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer to the public” in relation to any of our common shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any of our common shares to be offered so as to enable an investor to decide to purchase any of our common shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom. The underwriter has represented and agreed that:
|
● |
it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (FSMA)) received by it in connection with the issue or sale of our common shares in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
|
● |
it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to our common shares in, from or otherwise involving the United Kingdom. |
Switzerland. Our common shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (SIX) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to our common shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, or our common shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of our common shares will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA), and the offer of our common shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (CISA). Accordingly, no public distribution, offering or advertising, as defined in CISA, its implementing ordinances and notices, and no distribution to any non-qualified investor, as defined in CISA, its implementing ordinances and notices, shall be undertaken in or from Switzerland, and the investor protection afforded to acquirers of interests in collective investment schemes under CISA does not extend to acquirers of our common shares.
Australia. No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (ASIC) in relation to the offering.
This prospectus supplement does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act of 2001 (Corporations Act) and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of our common shares may only be made to persons (Exempt Investors) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer our common shares without disclosure to investors under Chapter 6D of the Corporations Act.
Our common shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring our common shares must observe such Australian on-sale restrictions.
This prospectus supplement contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus supplement is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
LEGAL MATTERS
The validity of the common shares offered hereby by us will be passed upon for us by Pushor Mitchell LLP, Kelowna, British Columbia, Canada, relating to matters of British Columbia law, and Fox Rothschild LLP, New York, New York, relating to matters of New York law. Faegre Drinker Biddle & Reath LLP, Minneapolis, Minnesota, is acting as counsel for the underwriter in connection with this offering.
EXPERTS
The consolidated financial statements incorporated into this prospectus supplement by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 have been audited by Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm. Their report, which is incorporated herein by reference, expresses an unqualified opinion on the consolidated financial statements. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference the information we file with them. This allows us to disclose important information to you by referencing those filed documents. We have previously filed the documents set forth below with the SEC and are incorporating them by reference into this prospectus supplement. Our SEC file no. is 001-36291.
● Annual Report on Form 10-K for the year ended December 31, 2018;
|
● |
Definitive Proxy Statement for our 2019 General and Special Meeting of Shareholders as filed with the SEC on April 8, 2019 (but only with respect to information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2018); |
|
● |
Quarterly Report on Form 10-Q for the quarter ended March 31, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended June 30, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended September 30, 2019; |
|
● |
Current Reports on Form 8-K (only to the extent information is “filed” and not “furnished”) filed with the SEC on January 3, 2019, January 9, 2019, February 26, 2019, May 23, 2019, June 4, 2019, June 19, 2019, June 21, 2019, August 13, 2019, October 30, 2019, January 3, 2020 and February 11, 2020; and |
|
● |
the description of our common shares contained in our Amendment No. 1 to our registration statement on Form 8-A that we filed with the SEC on June 4, 2019, and any amendment or report filed for the purpose of updating this description. |
We also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus supplement is a part and before the effective date of the registration statement and after the date of this prospectus supplement until the termination of the offering. The most recent information that we file with the SEC automatically updates and supersedes more dated information.
You may access our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statement, and amendments, if any, to those documents filed or furnished pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act with the SEC free of charge at the SEC’s website at www.sec.gov or our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
You can obtain a copy of any documents which are incorporated by reference in this prospectus supplement or prospectus supplement, except for exhibits which are not specifically incorporated by reference into those documents, at no cost, by writing or telephoning us at:
DiaMedica Therapeutics Inc.
Two Carlson Parkway, Suite 260
Minneapolis, Minnesota 55447
Attention: Secretary
(763) 312-6755
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered by this prospectus supplement. When used in this prospectus supplement, the term “registration statement” includes amendments to the registration statement as well as the exhibits, schedules, financial statements and notes filed as part of the registration statement. This prospectus supplement and the accompanying prospectus, which constitutes a part of the registration statement, does not contain all of the information in the registration statement. This prospectus supplement and accompanying prospectus omits information contained in the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and the common shares and other securities that may be offered by this prospectus supplement, reference is made to the registration statement. Statements herein concerning the contents of any contract or other document are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed with the SEC as an exhibit to the registration statement, each such statement being qualified by and subject to such reference in all respects.
In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at the SEC’s public reference room at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information about its public reference facilities and their copy charges.
We also file annual audited and interim unaudited financial statements, proxy statements and other information with the Ontario, Manitoba, Québec, Alberta and British Columbia Securities Commissions. Copies of these documents that are filed through the System for Electronic Document Analysis and Retrieval of the Canadian Securities Administrators are available at its website http://www.sedar.com.
In addition, we maintain a website that contains information regarding our company, including copies of reports, proxy statements and other information we file with the SEC. The address of our website is www.diamedica.com. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
PROSPECTUS

$50,000,000
Common Shares
Warrants
Units
We may from time to time offer to sell any combination of common shares, warrants and units described in this prospectus in one or more offerings. The aggregate initial offering price of all securities sold under this prospectus will not exceed $50,000,000.
This prospectus provides a general description of the securities that we may offer. Each time we sell securities, we will provide the specific terms of the securities offered in a supplement to this prospectus. The prospectus supplement may also add, update or change information contained in this prospectus. You should read this prospectus and the applicable prospectus supplement carefully before you invest in any securities. This prospectus may not be used to consummate a sale of securities unless accompanied by the applicable prospectus supplement.
We may from time to time offer and sell our securities in one offering or in separate offerings, to or through underwriters, dealers and agents or directly to purchasers. If any agents or underwriters are involved in the sale of any of these securities, the applicable prospectus supplement will provide the names of the agents or underwriters and any applicable fees, commissions or discounts.
Our common shares are listed on The Nasdaq Capital Market under the symbol “DMAC.” On December 31, 2019, the closing price of our common shares as reported on The Nasdaq Capital Market was $4.85 per share. As of the date of this prospectus, the aggregate market value of our common shares held by non-affiliates pursuant to General Instruction I.B.6 of Form S-3 is $52,130,710, which is calculated based on 10,748,600 common shares outstanding held by non-affiliates and a price of $4.85 per share, the closing price of our common shares on December 31, 2019, as reported on The Nasdaq Capital Market. During the prior 12 calendar month period that ends on and includes the date hereof, we have not offered or sold any of our common shares or other securities pursuant to General Instruction I.B.6 to Form S-3. Pursuant to General Instruction I.B.6 to Form S-3, in no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million.
We are an “emerging growth company,” as defined under federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements. See “About the Company – Implications of Being an Emerging Growth Company and Smaller Reporting Company” beginning on page 3 of this prospectus.
Investing in our securities involves risks. You should consider carefully the risks and uncertainties set forth in the section entitled “Risk Factors” beginning on page 4 of this prospectus, in the related prospectus supplement, and in the documents we file with the Securities and Exchange Commission that are incorporated by reference in this prospectus before making a decision to purchase our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is January 9, 2020
TABLE OF CONTENTS
Page
|
ABOUT THIS PROSPECTUS |
1 |
|
ABOUT THE COMPANY |
2 |
|
RISK FACTORS |
4 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
4 |
|
USE OF PROCEEDS |
5 |
|
DILUTION |
5 |
|
DESCRIPTION OF OUR COMMON SHARES |
6 |
|
DESCRIPTION OF WARRANTS |
11 |
|
DESCRIPTION OF UNITS |
12 |
|
PLAN OF DISTRIBUTION |
13 |
|
LEGAL MATTERS |
14 |
|
EXPERTS |
14 |
|
WHERE YOU CAN FIND MORE INFORMATION |
15 |
|
INCORPORATION OF DOCUMENTS BY REFERENCE |
15 |
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the United States Securities and Exchange Commission (SEC) utilizing a “shelf” registration process. Under this shelf registration process, we may offer and sell any combination of the securities described in this prospectus in one or more offerings up to a total dollar amount of $50,000,000.
This prospectus provides you with a general description of the respective securities that we may offer. Each time we sell securities under this shelf registration statement, we will provide a prospectus supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus. To the extent that any statement that we make in a prospectus supplement is inconsistent with statements made in this prospectus, the statements made in this prospectus will be deemed modified or superseded by those made in the prospectus supplement. You should read both this prospectus and the accompanying prospectus supplement, including all documents incorporated herein or therein by reference, together with additional information described under “Where You Can Find More Information” and “Incorporation of Documents by Reference.”
We have not authorized any dealer, salesperson or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus and the accompanying prospectus supplement. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or the accompanying prospectus supplement. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and the accompanying prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus and the accompanying prospectus supplement is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.
This prospectus may not be used to offer and sell securities unless it is accompanied by an additional prospectus or a prospectus supplement.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “DiaMedica,” “the Company,” “we,” “us,” “our” or similar references mean DiaMedica Therapeutics Inc. and its subsidiaries. References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value per share.
All references in this prospectus to “$,” “U.S. Dollars” and “dollars” are to United States dollars.
We own various unregistered trademarks and service marks, including our corporate logo. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the owner of such trademarks and trade names will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
ABOUT THE COMPANY
Overview
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant proteins. Our goal is to use our trade secrets and patented and licensed technologies to establish our company as a leader in the development and commercialization of therapeutic treatments derived from novel recombinant proteins. Our current focus is on chronic kidney disease (CKD) and acute ischemic stroke (AIS). We plan to advance DM199, our lead drug candidate, through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for CKD and AIS.
DM199 is a recombinant form of human tissue kallikrein-1 (KLK1). KLK1 is a serine protease (protein) produced primarily in the kidneys, pancreas and salivary glands that plays a critical role in the regulation of local blood flow and vasodilation (the widening of blood vessels, which decreases blood pressure) in the body, as well as an important role in inflammation and oxidative stress (an imbalance between potentially damaging reactive oxygen species, or free radicals, and antioxidants in your body). We believe DM199 has the potential to treat a variety of diseases where healthy functioning requires sufficient activity of KLK1 and its system, the kallikrein-kinin system (KKS).
AIS and CKD patients suffer from a lack of blood flow to the brain and kidneys, respectively. These patients also tend to exhibit lower than normal levels of endogenous KLK1. We believe treatment with DM199 could replenish low levels of endogenous KLK1, thereby releasing physiological levels of bradykinin (BK) when and where needed, generating beneficial nitric oxide and prostacyclin, setting in motion metabolic pathways that can improve blood flow (through vasoregulation) to damaged end-organs, such as the brain and kidneys, supporting structural integrity and normal functioning.
Today, forms of KLK1 derived from human urine and porcine pancreas are sold in Japan, China and Korea to treat AIS, CKD, retinopathy, hypertension and related vascular diseases. We believe millions of patients have been treated with these KLK1 therapies, and the data from more than 100 published papers and studies support their clinical benefit. However, there are numerous regulatory, commercial, and clinical drawbacks associated with KLK1 derived from human urine and porcine pancreas that can be overcome by developing a synthetic version of KLK1 such as DM199. We believe regulatory drawbacks are the primary reason why KLK1 derived from human urine and porcine pancreas are not currently available and used in the United States or Europe. We are not aware of any synthetic version of KLK1 with regulatory approval for human use in any country, nor are we aware of any synthetic version in development other than our drug candidate DM199. We believe at least five companies have attempted to create a synthetic version of KLK1, but have been unsuccessful.
In July 2019, we completed a Phase Ib clinical trial of DM199 in participants with moderate or severe CKD caused by Type I or Type II diabetes. We initiated dosing patients in this study in February 2019 and completed enrollment in July 2019. The study was performed to assess the pharmacokinetics (PK) of three dose levels of DM199 (3, 5 and 8 µg/kg), administered in a single subcutaneous dose, as well as the evaluation of safety, tolerability and secondary pharmacodynamic (PD) endpoints. The study results demonstrated that at the 3µg/kg dose level, the PK profiles were similar between moderate and severe CKD patients, and consistent with healthy subjects (normal kidney function) tested previously, and that DM199 was well tolerated with no dose-limiting tolerability. There were no deaths, no discontinuations due to a treatment-related adverse event (AE), and no treatment-related significant adverse events (SAEs). AEs were minor and consistent with standard treatment(s) in the CKD patient population. In addition, favorable overall PD results were also observed, including short-term improvements in Nitric Oxide (NO), average increase of 35.2%, Prostaglandin E2 (PGE2), average increase of 41.2%, estimated glomerular flow rate (eGFR), average increase of 4.08 mL/min/1732, and the urinary albumin to creatinine ratio (UACR), average decrease of 18.7%. PD results appeared to be drug related in that the greatest improvements occurred approximately 24 hours after DM199 administration and subsequently declined.
In December 2019, we began enrolling patients in a Phase II CKD trial named REDUX, Latin for restore, a multi-center, open-label investigation of approximately 60 participants with CKD, who are being enrolled in two cohorts (30 per cohort). The study is being conducted in the United States at up to 10 sites and will be focused on participants with CKD. Cohort I of the study is focused on non-diabetic, hypertensive African Americans with Stage II or III CKD. African Americans are at greater risk for CKD than Caucasians, and those who have the APOL1 gene mutation are at an even higher risk. The study is designed to capture the APOL1 gene mutation as an exploratory biomarker in this cohort. Cohort II of the study is focused on participants with IgA Nephropathy (IgAN). The study will evaluate two dose levels of DM199 within each cohort. Study participants will receive DM199 by subcutaneous injection twice weekly for 95 days. The primary study endpoints include safety, tolerability, blood pressure, proteinuria and kidney function, which will be evaluated by changes from baseline in eGFR and albuminuria, as measured by the UACR.
In October 2019, we completed enrollment in the REMEDY trial, the Company’s Phase II study assessing the safety, tolerability and markers of therapeutic efficacy of DM199 in participants suffering from AIS. Final enrollment was 92 participants. The markers of therapeutic efficacy will include multiple plasma-based biomarkers (e.g. C-reactive protein), the Modified Rankin Scale, National Institute of Health Stroke Scale and the Barthel Index. These markers are assessed at multiple points throughout the study, including 90 days post-stroke.
Implications of Being an Emerging Growth Company and Smaller Reporting Company
As a company with less than $1.07 billion of revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act), and we may remain an emerging growth company for up to five years from December 31, 2018. However, if certain events occur prior to the end of such five-year period, including if we become a large accelerated filer, our annual gross revenue exceeds $1.07 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. In particular, we are required to provide only two years of audited financial statements and are not required to disclose all of the executive compensation related information that would be required if we were not an emerging growth company. Accordingly, the information contained in our SEC reports may be different than the information you receive from other public companies in which you hold equity interests. However, we have irrevocably elected not to avail ourselves of the extended transition period for complying with new or revised accounting standards, and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We are also a “smaller reporting company” as defined in the United States Securities Exchange Act of 1934, as amended (Exchange Act). We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies until the fiscal year following the determination that our voting and non-voting common shares held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are more than $100 million during the most recently completed fiscal year and our voting and non-voting common shares held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter.
Corporate Information
Our principal executive offices are located at 2 Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447. Our telephone number is (763) 312-6755, and our Internet website address is www.diamedica.com. We make available on our website free of charge a link to our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as practicable after we electronically file such material with the SEC. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We are a corporation governed under the British Columbia Business Corporations Act. Our company was initially incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of incorporation dated January 21, 2000. Our articles were amended (i) on February 26, 2001 to change our corporate name to DiaMedica Inc., (ii) on April 11, 2016 to continue the Company from The Corporations Act (Manitoba) to the CBCA, (iii) on December 28, 2016 to change our corporate name to DiaMedica Therapeutics Inc., (iv) on September 24, 2018 to permit us to hold shareholder meetings in the U.S. and to permit our directors, between annual general meetings of our shareholders, to appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of shareholders, (v) on November 15, 2018 to effect a 1-for-20 consolidation of our common shares, and (vi) on May 31, 2019, to continue our existence from a corporation incorporated under the Canada Business Corporations Act into British Columbia under British Columbia’s Business Corporations Act.
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described in our filings with the SEC referred to under the heading “Where You Can Find More Information,” including the risk factors incorporated by reference herein from our most recent annual report on Form 10-K and quarterly reports on Form 10-Q and from other reports and documents we file with the SEC after the date of this prospectus that are incorporated by reference herein, together with all of the other information included in this prospectus, the applicable prospectus supplement and the documents we incorporate by reference.
If any of these risks were to occur, our business, financial condition, results of operations or cash flows could be adversely affected. You could lose all or part of your investment. When we offer and sell any securities pursuant to a prospectus supplement, we may include additional risk factors relevant to that offering in the prospectus supplement.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this prospectus and the related prospectus supplement that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology, and the use of future dates.
The forward-looking statements in or incorporated by reference into this prospectus or the related prospectus supplement may include, among other things, statements about:
|
● |
our plans to develop, obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of CKD and AIS and our expectations regarding the benefits of our DM199 product candidate; |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain required regulatory approvals of our DM199 product candidate for CKD and AIS; |
|
● |
the perceived benefits of our DM199 product candidate over existing treatment options for CKD and AIS; |
|
● |
the potential size of the markets for our DM199 product candidate and our ability to serve those markets; |
|
● |
the rate and degree of market acceptance, both in the United States and internationally, of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for and commercialize our DM199 product candidate for CKD and AIS, and any adverse ramifications as a result of our termination of a license and collaboration agreement with Ahon Pharmaceutical Co., Ltd.; |
|
● |
the success, cost and timing of planned clinical trials, as well as our reliance on collaboration with third parties to conduct our clinical trials; |
|
● |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
● |
expectations regarding federal, state, and foreign regulatory requirements and developments, such as potential United States Food and Drug Administration (FDA) regulation of our DM199 product candidate for CKD and AIS; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain funding for our operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for our DM199 product candidate for CKD and AIS; |
|
● |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; and |
|
● |
our anticipated use of the net proceeds from our December 2018 initial public offering in the United States and any offering under this prospectus and the related prospectus supplement to be filed in connection with such offering. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under “Risk Factors” in this prospectus and related prospectus supplement. Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Except as required by law, including the securities laws of the United States, we do not intend to update any forward-looking statements to conform these statements to actual results or to changes in our expectations.
USE OF PROCEEDS
Unless otherwise indicated in the prospectus supplement, we intend to use the net proceeds from the sale of securities and any exercise of warrants under this prospectus and related prospectus supplement to continue our clinical and product development activities and for other working capital and general corporate purposes. The prospectus supplement relating to a particular offering of securities by us will identify the use of proceeds for that offering. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the proceeds. Pending the uses described above, we intend to deposit the proceeds in our non-interest bearing checking account, U.S. Treasury money market fund or invest them temporarily in short-term or marketable securities until we use them for their stated purpose.
DILUTION
We will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors purchasing securities in an offering under this prospectus:
|
● |
the net tangible book value per share of our equity securities before and after the offering; |
|
● |
the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and |
|
● |
the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers. |
DESCRIPTION OF OUR COMMON SHARES
General
The following is a summary of the material terms of our common shares, as well as other material terms of our Notice of Articles and Articles and certain provisions of the British Columbia Business Corporations Act (BCBCA). References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value. This summary does not purport to be complete and is qualified in its entirety by the provisions of our Notice of Articles and Articles, which are included as exhibits to the registration statement of which this prospectus forms a part. For more information on how you can obtain our Notice of Articles and Articles, see the heading “Where You Can Find Additional Information.”
Authorized Share Capital
We have an authorized share capital consisting of an unlimited number of common shares, no par value per share.
Outstanding Common Shares
As of December 31, 2019, there were 12,006,874 common shares issued and outstanding. As of December 31, 2019, the following additional common shares were reserved for issuance:
|
● |
766,953 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.65 per share; |
|
● |
605,181 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.09 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
615,178 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.55 per share; and |
|
● |
1,384,822 common shares were reserved for future issuance in connection with future grants under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
Certain Rights of the Common Shares
Dividends
Holders of our common shares are entitled to share pro rata in such dividends as may be declared by our Board of Directors. Pursuant to the provisions of the BCBCA, we may not declare or pay a dividend if there are reasonable grounds for believing that we are, or would after the payment be, unable to pay our liabilities as they become due in the ordinary course of business. We may pay a dividend by issuing fully paid shares, bonds, debentures or other of our securities or in property (including money).
Liquidation, Dissolution or Winding-Up
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company or any other distribution of our assets among our shareholders for the purpose of winding-up our affairs, holders of common shares are entitled to share pro rata in our assets available for distribution after we pay our creditors.
Voting Rights and Shareholders’ Meetings
Holders of our common shares are entitled to receive notice of and to attend and vote at all meetings of our shareholders. Each holder of our common shares is entitled to one vote, either in person or by proxy, on all matters submitted to shareholders.
Our Board of Directors must call an annual general meeting of shareholders to be held not later than 15 months after the last preceding annual general meeting of shareholders but no later than six months after the end of our preceding financial year end and may, at any time, call a special meeting of shareholders. Under our articles, a meeting of our shareholders may be held anywhere in or outside of British Columbia, as determined by the Board of Directors. For purposes of determining the shareholders who are entitled to receive notice of or to vote at a meeting of shareholders, the Board of Directors may, in accordance with National Instrument 54-101 - Communications with Beneficial Owners of Securities of a Reporting Issuer of the Canadian Securities Administrators, fix in advance a date as the record date for that determination of shareholders, but that record date may not be more than 60 days or less than 30 days before the date on which the meeting is to be held.
Our Articles provide that notice of the time and place of a meeting of shareholders must be sent to each shareholder entitled to vote at the meeting, each director and to our auditors, not more than 50 days and not less than 21 days prior to the meeting. Under our Articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding not less than 33 1/3 of the issued common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting. A shareholder may participate in a meeting by means of telephone or other communication medium that permits all persons participating in the meeting to communicate with each other during the meeting.
In the case of joint shareholders, one of the holders present at a meeting, either personally or by proxy, may, in the absence of the other holder(s) of the shares, vote the shares. If two or more joint shareholders are present, personally or by proxy, then only the vote of the joint shareholder present whose name stands first on the central securities register in respect of the share will be counted.
No Preemption Rights; Limited Restrictions on Directors’ Authority to Issue Common Shares
Existing holders of our common shares have no rights of preemption or first refusal under our Articles or the BCBCA with respect to future issuances of our common shares. The common shares do not have conversion rights, are not subject to redemption and do not have the benefit of any sinking fund provisions. Subject to the rules and policies of The Nasdaq Stock Market and applicable corporate and securities laws, our Board of Directors has the authority to issue additional common shares.
Amendments to Articles
The Articles and the BCBCA govern the rights of holders of our common shares.
Subject to the BCBCA, unless an alteration to the Company’s Notice of Articles would be required, our directors can authorize the alteration of our Articles to, among other things, create additional classes or series of shares or, if none of the shares of a class or series are allotted or issued, eliminate that class or series of shares.
Subject to the BCBCA, our shareholders can authorize the alteration of our Articles and Notice of Articles to create or vary the rights or restrictions attached to any class of our shares by passing an ordinary resolution at a duly convened meeting of shareholders. An alteration to the Company’s Notice of Articles will not be effective until the notice of alteration is filed with the registrar pursuant to the BCBCA. An alteration to the Company’s Articles, which is not an alteration to the Company’s Notice of Articles, will be effective on the date and time that the resolution is received for deposit at the Company’s records office.
Fundamental Changes
Pursuant to the BCBCA, we may not effect any of the following fundamental changes without the consent of the holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and separately as a class at a duly convened meeting of our shareholders:
|
● |
any proposed amalgamation involving our company in respect of which the BCBCA requires that the approval of our shareholders be obtained; |
|
● |
any proposed plan of arrangement pursuant to the BCBCA involving our company in respect of which the BCBCA or any order issued by an applicable court requires that the approval of our shareholders be obtained; |
|
● |
any proposed sale, lease or exchange of all or substantially all of our undertaking; and |
|
● |
any voluntary liquidation of our company. |
Election and Removal of Directors
At each annual general meeting of shareholders, our shareholders are required to elect directors to hold office for a term expiring not later than the close of the next annual general meeting of shareholders. Our Board of Directors may fill vacancies among the Board. Our directors may also, between annual general meetings of our shareholders, appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third (1/3) of the number of directors who held office at the expiration of the last meeting of shareholders.
Since shareholders do not have cumulative voting rights, holders of more than 50% of our outstanding common shares can elect all of our directors if they choose to do so. In such event, holders of the remaining shares will be unable to elect any director.
Under the BCBCA, a public company must have a minimum of three directors, who are not required to be resident Canadians.
Under the BCBCA, a director may be removed by shareholders by special resolution unless the Articles provide for a lower approval level. The Articles allow shareholders to remove directors by a special resolution if approved by holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and voting separately as a class at a duly convened meeting of our shareholders.
Registration Rights
We have not granted any rights to have our common shares or other securities registered under the United States Securities Act of 1933, as amended (Securities Act).
Listing
Our common shares are listed and trade in the United States on The Nasdaq Capital Market under the trading symbol “DMAC.”
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
Limitation of Liability and Indemnification Matters
Our Articles provide that we will indemnify our directors, former directors, his or her heirs and legal personal representatives and other individuals as we may determine against all eligible penalties to which such person is or may be liable to the fullest extent permitted by British Columbia law. We will pay all expenses actually and reasonably incurred by such person, either as such expenses are incurred in advance of the final disposition of an eligible proceeding or after the final disposition of an eligible proceeding. British Columbia law provides that a company must not indemnify its directors if any of the following circumstances apply:
|
● |
if the indemnity or payment is made under an earlier agreement to indemnify or pay expenses and, at the time that the agreement to indemnify or pay expenses was made, the company was prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if the indemnity or payment is made otherwise than under an earlier agreement to indemnify or pay expenses and, at the time that the indemnity or payment is made, the company is prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if, in relation to the subject matter of the relevant proceeding, the director did not act honestly and in good faith with a view to the best interests of the company or the associated corporation, as the case may be, with such associated corporation being an affiliate of the company or a partnership, trust, joint venture or other unincorporated entity in which the director served in the capacity as a director or a position equivalent to that thereof, at the request of the company; or |
|
● |
in the case of the relevant proceeding other than a civil proceeding, if the director did not have reasonable grounds for believing that the director’s conduct in respect of which the proceeding was brought was lawful. |
Notwithstanding any of the above prohibitions, the company or a director may apply to court for an order that the company must indemnify the director for any liability or expenses incurred by the director or for any other related obligations of the company.
The Articles also permit us to purchase insurance on behalf of any officer, director, employee or other agent of our company, of an affiliated entity, or, at our request, of another entity, for any liability arising out of that person’s actions in such capacity. We have entered into indemnification agreements with each of our current directors and executive officers requiring us to indemnify these individuals to the fullest extent permitted under British Columbia law against liability that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified, and have received a written undertaking from each such director and officer as required under British Columbia law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Shareholder Rights Plan
We adopted a shareholder rights plan agreement (Rights Plan). The Rights Plan is designed to provide adequate time for the Board of Directors and the shareholders to assess an unsolicited takeover bid for DiaMedica, to provide the Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The Rights Plan was renewed at the Company’s annual general meeting of shareholders in December 2017 and is set to expire at the close of the Company’s annual general meeting of shareholders in 2020.
The rights issued under the Rights Plan will initially attach to and trade with the common shares, and no separate certificates will be issued unless an event triggering these rights occurs. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of the outstanding common shares without complying with the “Permitted Bid” provisions of the Rights Plan or without approval of the Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time.
Under the Rights Plan, a Permitted Bid is a bid made to all holders of the common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
The issuance of common shares upon the exercise of the rights is subject to receipt of certain regulatory approvals.
Anti-takeover Laws
In Canada, takeover bids are governed by provincial corporate and securities laws and the rules of applicable stock exchanges. The following description of the rules relating to acquisitions of securities and takeover bids to which Canadian corporate and securities laws apply does not purport to be complete and is subject, and qualified in its entirety by reference, to applicable corporate and securities laws, which may vary from province to province.
A party (acquiror) who acquires beneficial ownership of, or control or direction over, more than 10% of the voting or equity securities of any class of a reporting issuer (or securities convertible into voting or equity securities of any class of a reporting issuer) will generally be required to file with applicable provincial regulatory authorities both a news release and a report containing the information prescribed by applicable securities laws. Subject to the below, the acquiror (including any party acting jointly or in concert with the acquiror) will be prohibited from purchasing any additional securities of the class of the target company previously acquired for a period commencing on the occurrence of an event triggering the aforementioned filing requirement and ending on the expiry of one business day following the filing of the report. This filing process and the associated restriction on further purchases also apply in respect of subsequent acquisitions of 2% or more of the securities of the same class (or securities convertible into voting or equity securities of any class of a reporting issuer). The restriction on further purchases does not apply to an acquiror that beneficially owns, or controls or directs, 20% or more of the outstanding securities of that class.
In addition to the foregoing, certain other Canadian legislation may limit a Canadian or non-Canadian entity’s ability to acquire control over or a significant interest in us, including the Competition Act (Canada) and the Investment Canada Act (Canada). Issuers may also approve and adopt shareholder rights plans or other defensive tactics designed to be triggered upon the commencement of an unsolicited bid and make the company a less desirable takeover target.
DESCRIPTION OF WARRANTS
The following summary of the general terms and provisions of the warrants represented by warrant agreements and warrant certificates that we may offer using this prospectus and a prospectus supplement is only a summary and does not purport to be complete. You must look at the applicable forms of warrant agreement and warrant certificate for a full understanding of the specific terms of any warrant. The forms of the warrant agreement and the warrant certificate will be filed or incorporated by reference as exhibits to the registration statement to which this prospectus is a part. See “Where You Can Find More Information” for information on how to obtain copies.
A prospectus supplement will describe the specific terms of the warrants offered under that prospectus supplement, including any of the terms in this section that will not apply to those warrants, and any special considerations, including tax considerations, applicable to investing in those warrants.
General
We may issue warrants to purchase common shares alone or together with other securities offered by the applicable prospectus supplement. The warrants may be issued independently or together with any securities and may be attached to or separate from the securities. We may enter into a warrant agreement with a warrant agent. If we elect to do so, the warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency or trust for or with any registered holders of warrants or beneficial owners of warrants.
The prospectus supplement relating to any warrants we offer will describe the specific terms relating to the offering. These terms may include some or all of the following:
|
● |
the offering price; |
|
● |
the currencies in which the warrants will be offered; |
|
● |
the total number of shares that may be purchased if all of the holders exercise the warrants; |
|
● |
the number of shares that may be purchased if a holder exercises any one warrant and the price at which and currencies in which shares may be purchased upon exercise; |
|
● |
the date on and after which the holder of the warrants can transfer them separately from the related underlying common shares; |
|
● |
the date on which the right to exercise the warrants begins and expires; |
|
● |
the triggering event and the terms upon which the exercise price and the number of underlying common shares that the warrants are exercisable into may be adjusted; |
|
● |
whether the warrants will be issued in registered or bearer form; |
|
● |
the identity of any warrant agent with respect to the warrants and the terms of the warrant agency agreement with that warrant agent; |
|
● |
a discussion of material U.S. federal income tax consequences; and |
|
● |
any other terms of the warrants. |
A holder of warrants may:
|
● |
exchange them for new warrants of different denominations; |
|
● |
present them for registration of transfer, if they are in registered form; and |
|
● |
exercise them at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement. |
Until the warrants are exercised, holders of the warrants will not have any of the rights of holders of the underlying common shares.
Exercise of Warrants
Each holder of a warrant is entitled to purchase the number of common shares at the exercise price described in the applicable prospectus supplement. After the close of business on the day when the right to exercise terminates (or a later date if we extend the time for exercise), unexercised warrants will become void.
Holders of warrants may exercise them by:
|
● |
delivering to the warrant agent the payment required to purchase the underlying common shares, as stated in the applicable prospectus supplement; |
|
● |
properly completing and signing the reverse side of their warrant certificate(s), if any, or other exercise documentation; and |
|
● |
delivering their warrant certificate(s), if any, or other exercise documentation to the warrant agent within the time specified by the applicable prospectus supplement. |
If you comply with the procedures described above, your warrants will be considered to have been exercised when the warrant agent receives payment of the exercise price. As soon as practicable after you have completed these procedures, we will issue and deliver to you the common shares that you purchased upon exercise. If you exercise fewer than all of the warrants represented by a warrant certificate, we will issue to you a new warrant certificate for the unexercised amount of warrants.
Amendments and Supplements to Warrant Agreements
We may amend or supplement a warrant agreement or warrant certificates without the consent of the holders of the warrants if the changes are not inconsistent with the provisions of the warrants and do not adversely affect the interests of the holders.
DESCRIPTION OF UNITS
We may, from time to time, issue units comprised of one or more of the other securities described in this prospectus in any combination. A prospectus supplement will describe the specific terms of the units offered under that prospectus supplement, and any special considerations, including tax considerations, applicable to investing in those units. You must look at the applicable prospectus supplement and any applicable unit agreement for a full understanding of the specific terms of any units. The form of unit agreement will be filed or incorporated by reference as an exhibit to the registration statement to which this prospectus is a part. See “Where You Can Find More Information” for information on how to obtain copies.
PLAN OF DISTRIBUTION
We may sell the securities from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination of these methods. We may sell the securities separately or together:
|
● |
through one or more underwriters or dealers in a public offering and sale by them; |
|
● |
through agents; and/or |
|
● |
directly to one or more purchasers. |
We may distribute the securities from time to time in one or more transactions:
|
● |
at a fixed price or prices, which may be changed; |
|
● |
at market prices prevailing at the time of sale; |
|
● |
at prices related to such prevailing market prices; or |
|
● |
at negotiated prices. |
We may solicit directly offers to purchase the respective securities being offered by this prospectus. We may also designate agents to solicit offers to purchase the respective securities from time to time. We will name in a prospectus supplement any agent involved in the offer or sale of our securities. If we utilize a dealer in the sale of the respective securities being offered by this prospectus, we will sell the respective securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. If we utilize an underwriter in the sale of the respective securities being offered by this prospectus, we will execute an underwriting agreement with the underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement that the underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we or the purchasers of securities for whom the underwriter may act as agent may compensate the underwriter in the form of underwriting discounts or commissions. In connection with the offering of securities, we may grant to the underwriters an option to purchase additional securities with an additional underwriting commission, as may be set forth in the accompanying prospectus supplement. If we grant any such option, the terms of such option will be set forth in the prospectus supplement for such securities. The underwriter may sell the securities to or through dealers, and the underwriter may compensate those dealers in the form of discounts, concessions or commissions. No Financial Industry Regulatory Authority (FINRA) member firm may receive compensation in excess of that allowable under FINRA rules, including Rule 5110, in connection with the offering of the securities.
We will provide in the applicable prospectus supplement any compensation we will pay to underwriters, dealers or agents in connection with the offering of the respective securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect thereof.
Our common shares are currently listed on The Nasdaq Capital Market. The other securities that may be offered under this prospectus and the related prospectus supplement may or may not be listed on a national securities exchange. To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. The transactions may be discontinued at any time.
We may authorize underwriters, dealers or agents to solicit offers by certain purchasers to purchase the respective securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. The contracts will be subject only to those conditions set forth in the prospectus supplement, and the prospectus supplement will set forth any commissions we pay for solicitation of these contracts.
We may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, the parties may sell securities covered by this prospectus and the applicable prospectus supplement, including short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be identified in the applicable prospectus supplement or a post-effective amendment to this registration statement. In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
The anticipated date of delivery of offered securities will be set forth in the applicable prospectus supplement relating to each offer.
LEGAL MATTERS
Unless the applicable prospectus supplement indicates otherwise, the validity of the securities in respect of which this prospectus is being delivered will be passed upon for us by Pushor Mitchell LLP, Kelowna, British Columbia, Canada, relating to matters of British Columbia or Canadian law, and Fox Rothschild LLP, New York, New York, relating to matters of New York law. Additional legal matters may be passed upon for us or any underwriters, dealers or agents by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements incorporated into this prospectus by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 have been audited by Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm. Their report, which is incorporated herein by reference, expresses an unqualified opinion on the consolidated financial statements. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at the SEC’s public reference room at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information about its public reference facilities and their copy charges.
We also file annual audited and interim unaudited financial statements, proxy statements and other information with the Ontario, Manitoba, Québec, Alberta and British Columbia Securities Commissions. Copies of these documents that are filed through the System for Electronic Document Analysis and Retrieval of the Canadian Securities Administrators are available at its website www.sedar.com.
In addition, we maintain a website that contains information regarding our company, including copies of reports, proxy statements and other information we file with the SEC. The address of our website is www.diamedica.com. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered by this prospectus. When used in this prospectus, the term “registration statement” includes amendments to the registration statement as well as the exhibits, schedules, financial statements and notes filed as part of the registration statement. This prospectus, which constitutes a part of the registration statement, does not contain all of the information in the registration statement. This prospectus omits information contained in the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and the common shares and other securities that may be offered by this prospectus, reference is made to the registration statement. Statements herein concerning the contents of any contract or other document are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed with the SEC as an exhibit to the registration statement, each such statement being qualified by and subject to such reference in all respects.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference the information we file with them. This allows us to disclose important information to you by referencing those filed documents. We have previously filed the documents set forth below with the SEC and are incorporating them by reference into this prospectus. Our SEC file no. is 001-36291.
|
● |
|
● |
|
● |
Quarterly Report on Form 10-Q for the quarter ended March 31, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended June 30, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended September 30, 2019; |
|
● |
Current Reports on Form 8-K (only to the extent information is “filed” and not “furnished”) filed with the SEC on January 3, 2019, January 9, 2019, February 26, 2019, May 23, 2019, June 4, 2019, June 19, 2019, June 21, 2019, August 13, 2019, and October 30, 2019; and |
|
● |
the description of our common shares contained in our Amendment No. 1 to our registration statement on Form 8-A that we filed with the SEC on June 4, 2019, and any amendment or report filed for the purpose of updating this description. |
We also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is a part and before the effective date of the registration statement and after the date of this prospectus until the termination of the offering. The most recent information that we file with the SEC automatically updates and supersedes more dated information.
You may access our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statement, and amendments, if any, to those documents filed or furnished pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act with the SEC free of charge at the SEC’s website at www.sec.gov or our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
You can obtain a copy of any documents which are incorporated by reference in this prospectus or prospectus supplement, except for exhibits which are not specifically incorporated by reference into those documents, at no cost, by writing or telephoning us at:
DiaMedica Therapeutics Inc.
Two Carlson Parkway, Suite 260
Minneapolis, Minnesota 55447
Attention: Secretary
(763) 312-6755
2,125,000 Common Shares

PROSPECTUS SUPPLEMENT
Craig-Hallum Capital Group
The date of this prospectus supplement is February 11, 2020