
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-235775
The information in this preliminary prospectus supplement is not complete and may be changed. A registration statement relating to these securities has become effective by rule of the Securities and Exchange Commission. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities, and we are not soliciting offers to buy these securities, in any state or other jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 5, 2020
PRELIMINARY PROSPECTUS SUPPLEMENT
(To Prospectus dated January 9, 2020)
Common Shares

__________________________________________
We are offering common shares, no par value per share.
Our common shares are listed on the Nasdaq Capital Market under the symbol “DMAC.” On August 4, 2020, the last reported sales price of our common shares on the Nasdaq Capital Market was $5.86 per share.
We are an “emerging growth company” and a “smaller reporting company” as defined under federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements. See “Prospectus Supplement Summary – Our Company – Implications of Being an Emerging Growth Company.”
__________________________________________
Investing in our common shares involves significant risks. Please read the information contained in or incorporated by reference under the heading “Risk Factors” beginning on page S-13 of this prospectus supplement, and under similar headings in other documents filed after the date hereof and incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
PER SHARE |
TOTAL |
|||||||
|
Public offering price |
$ | $ | ||||||
|
Underwriting discounts and commissions(1) |
$ | $ | ||||||
|
Proceeds, before expenses, to us |
$ | $ | ||||||
_______________
|
(1) |
We have agreed to reimburse the underwriters for certain expenses. We refer you to “Underwriting” beginning on page S-45 of this prospectus supplement for additional information regarding total underwriting compensation. |
Delivery of the common shares is expected to be made on or about , 2020
We have granted the underwriters an option for a period of 30 days to purchase an additional common shares. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
__________________________________________
Guggenheim Securities
Craig-Hallum Capital Group
________________________________________
The date of this prospectus supplement is , 2020
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
|
ABOUT THIS PROSPECTUS SUPPLEMENT |
S-1 |
|
INDUSTRY AND MARKET DATA |
S-2 |
|
PROSPECTUS SUPPLEMENT SUMMARY |
S-3 |
|
RISK FACTORS |
S-13 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
S-18 |
|
USE OF PROCEEDS |
S-20 |
|
DIVIDEND POLICY |
S-21 |
|
CAPITALIZATION |
S-22 |
|
DILUTION |
S-23 |
|
DESCRIPTION OF COMMON SHARES |
S-24 |
|
CERTAIN UNITED STATES INCOME TAX CONSIDERATIONS |
S-35 |
|
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS |
S-42 |
|
UNDERWRITING |
S-44 |
|
LEGAL MATTERS |
S-49 |
|
EXPERTS |
S-49 |
|
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE |
S-49 |
|
WHERE YOU CAN FIND MORE INFORMATION |
S-50 |
|
BASE PROSPECTUS |
|
ABOUT THIS PROSPECTUS |
1 |
|
ABOUT THE COMPANY |
2 |
|
RISK FACTORS |
4 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
4 |
|
USE OF PROCEEDS |
5 |
|
DILUTION |
5 |
|
DESCRIPTION OF OUR COMMON SHARES |
6 |
|
DESCRIPTION OF WARRANTS |
11 |
|
DESCRIPTION OF UNITS |
12 |
|
PLAN OF DISTRIBUTION |
13 |
|
LEGAL MATTERS |
14 |
|
EXPERTS |
14 |
|
WHERE YOU CAN FIND MORE INFORMATION |
15 |
|
INCORPORATION OF DOCUMENTS BY REFERENCE |
15 |
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus are part of a shelf registration statement on Form S-3 (File No. 333-235775) that we filed with the Securities and Exchange Commission (SEC) on January 2, 2020 and was declared effective by the SEC on January 9, 2020, pursuant to which we may from time to time offer various securities in one or more offerings.
This document is in two parts. The first part is this prospectus supplement, which describes the terms of this offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein or therein. The second part, the accompanying prospectus, including the documents incorporated by reference into the accompanying prospectus, provides more general information. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus or any document incorporated by reference herein or therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date — for example, a document incorporated by reference in the accompanying prospectus — the statement in the document having the later date modifies or supersedes the earlier statement.
Neither we nor the underwriters have authorized anyone to provide information different from that contained in this prospectus supplement, the accompanying prospectus and any free writing prospectus that we have authorized for use in this offering. If anyone provides you with different or inconsistent information, you should not rely on it. Neither we nor the underwriters take any responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither the delivery of this prospectus supplement, the accompanying prospectus and any free writing prospectus that we have authorized for use in this offering, nor the sale of our common shares means that information contained in this prospectus supplement, the accompanying prospectus and any free writing prospectus that we have authorized for use in this offering, is correct after their respective dates. It is important for you to read and consider all information contained in this prospectus supplement and the accompanying prospectus, including the information incorporated by reference into this prospectus supplement, the accompanying prospectus and any free writing prospectus that we have authorized for use in connection with this offering in making your investment decision.
This prospectus supplement does not contain all of the information that is important to you. You should also read and consider the information in the documents to which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference” in this prospectus supplement. You should rely only on the information contained or incorporated by reference in this document. You should assume that the information in this prospectus supplement and the accompanying prospectus, as well as the information we have filed with the SEC and incorporated by reference in this document, is accurate only as of its date or the date which is specified in those documents.
We are offering to sell, and seeking offers to buy, and the underwriters are soliciting offers to buy, these securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of the securities in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
This prospectus supplement and the information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference herein are the property of their respective owners.
Unless otherwise indicated or the context otherwise requires, references in this prospectus supplement and the accompanying prospectus to “DiaMedica,” the “Company,” “we,” “us” and “our” refer to DiaMedica Therapeutics Inc. References in this prospectus supplement to “voting common shares” or “common shares” mean our voting common shares, no par value per share.
All references in this prospectus supplement to “$,” “U.S. Dollars” and “dollars” are to United States dollars.
INDUSTRY AND MARKET DATA
In addition to the industry, market and competitive position data referenced in this prospectus supplement from our own internal estimates and research, some market data and other statistical information included in this prospectus supplement are based in part upon information obtained from third-party industry publications, research, surveys and studies, none of which we commissioned. Third-party industry publications, research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
We are responsible for all of the disclosure in this prospectus supplement and while we believe that each of the publications, research, surveys and studies included in this prospectus supplement are prepared by reputable sources, neither we nor the underwriter has independently verified market and industry data from third-party sources. In addition, while we believe our internal company research and estimates are reliable, such research and estimates have not been verified by independent sources. Assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Cautionary Note Regarding Forward-Looking Statements.”
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights certain information about us, this offering and information appearing elsewhere in this prospectus supplement or the accompanying prospectus and in the documents we incorporate by reference herein and therein. This summary is not complete and does not contain all of the information that you should consider before investing in our securities. The following summary is qualified in its entirety by, and should be read in conjunction with, the more detailed information and financial statements and notes thereto appearing elsewhere in this prospectus supplement, the accompanying prospectus and in the documents incorporated by reference herein and therein. Before you decide to invest in our securities, to fully understand this offering and its consequences to you, you should read the entire prospectus supplement carefully, including the matters set forth under the caption “Risk Factors” beginning on page S-13 of this prospectus supplement and page 4 of the accompanying prospectus, and the consolidated financial statements and related notes included or incorporated by reference in this prospectus supplement, the accompanying prospectus and the other documents incorporated by reference herein and therein.
Our Company
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant, or synthetic, proteins. Our goal is to use our patented and licensed technologies to establish our company as a leader in the development and commercialization of therapeutic treatments from novel recombinant proteins. Our current focus is on chronic kidney disease (CKD) and acute ischemic stroke (AIS). We plan to advance DM199, our lead drug candidate, through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for CKD and AIS.
DM199 is a recombinant form of human tissue kallikrein-1 (KLK1). KLK1 is a serine protease (protein), produced primarily in the kidneys, pancreas and salivary glands, which plays a critical role in the regulation of local blood flow and vasodilation (the widening of blood vessels which decreases blood pressure) in the body, as well as an important role in inflammation and oxidative stress (an imbalance between potentially damaging reactive oxygen species, or free radicals, and antioxidants in the body). We believe DM199 has the potential to treat a variety of diseases where healthy functioning requires sufficient activity of KLK1 and its system, the kallikrein-kinin system (KKS).
CKD and AIS patients suffer from impaired blood flow to the kidneys and brain, respectively. These patients also tend to exhibit lower than normal levels of endogenous (produced by the body) KLK1. We believe treatment with DM199 could replenish levels of KLK1, thereby allowing the natural function of KKS to release bradykinin in the body where and when needed, generating beneficial nitric oxide and prostacyclin, setting in motion metabolic pathways that can improve blood flow (through vasoregulation), dampen inflammation and protect tissues and end-organs from ischemic damage, supporting structural integrity and normal functioning.
Today, forms of KLK1 derived from human urine and porcine pancreas are sold in Japan, China and Korea to treat AIS, CKD, retinopathy, hypertension and related vascular diseases. We believe millions of patients have been treated with these KLK1 therapies and the data from more than 100 published papers and studies support their clinical benefit. However, there are numerous regulatory, commercial and clinical drawbacks associated with KLK1 derived from human urine and porcine pancreas which can be overcome by developing a synthetic version of KLK1 such as DM199. We believe higher regulatory standards are the primary reason why KLK1 derived from human urine and porcine pancreas are not currently available and used in the United State or Europe. We are not aware of any synthetic version of KLK1 with regulatory approval for human use in any country, nor are we aware of any synthetic version in development other than our drug candidate, DM199.
As described in more detail below, positive top-line results from ReMEDy, a 92-subject study in acute ischemic stroke, including the achievement of primary safety and tolerability endpoints and no DM199-related serious adverse events, were announced in May 2020. In addition, there was also a demonstrated therapeutic effect in participants that received tissue plasminogen activator (tPA) prior to enrollment but not in participants receiving mechanical thrombectomy prior to enrollment according to top-line Phase II results.
We have conducted numerous internal and third-party analyses to evaluate the structural and functional performance of DM199 as compared to KLK1 derived from human urine. The results of these studies have demonstrated that DM199 is structurally and functionally equivalent to KLK1 derived from human urine in that (i) the amino acid structure of DM199 is identical to the human urine form, (ii) the enzymatic and pharmacokinetic profiles are substantially similar to human urinary derived KLK1 and (iii) the physiological effects of DM199 on blood pressure mirror that of human urinary derived KLK1. We believe that the results of this work suggest that the therapeutic action of DM199 will be the same or better than that of the forms of KLK1 marketed in Asia. In addition, we have completed enrollment in seven clinical trials with DM199 treating over 200 subjects, and the results have shown that DM199 has been safe and well-tolerated. However, DM199 has not been, and we cannot provide any assurance that it ultimately will be, determined to be safe or effective for purposes of granting marketing approval by the U.S. Food and Drug Administration (FDA) or any comparable agency.
Our recombinant form of DM199 is protected by issued composition of matter and delivery patents in the United States and Europe (expiration 2033); a pending worldwide patent (expiration 2038) that covers a range of DM199 dose levels and dosing regimens useful for treating a wide range of diseases associated with microvascular dysfunction; an exclusive license with our manufacturing partner for use of their cell line and proprietary expression system for manufacturing synthetic KLK1; and numerous trade-secrets. In addition, we believe DM199 cannot be reverse engineered to develop a copycat version of our therapy. This adds additional protection to our intellectual property, especially as we evaluate DM199 licensing.
Our Programs
The primary focus for our DM199 program development is currently on CKD and AIS. The current status of our product candidates in clinical development is as follows:
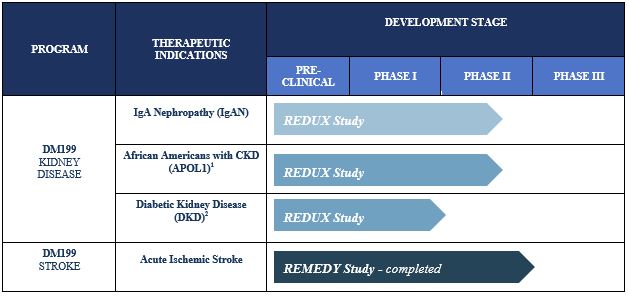
|
1. |
Due to the heightened risk of African Americans with the APOL1 genetic mutation to progress to end-stage renal disease, participants in this cohort will be tested for the presence of the APOL1 genetic mutation. |
|
2. |
Initiation of this cohort and development for this indication is contingent upon successful completion of this offering. |
Chronic Kidney Disease
CKD is a widespread health problem that generates significant economic burden throughout the world. According to the National Kidney Foundation, approximately 30 million Americans and 120 million Chinese suffer from this debilitating and potentially life-threatening condition. CKD is characterized by a progressive decline in overall kidney function, increasing the risk of premature death, cardiovascular events and hospitalization. End-stage renal disease (ESRD) is the final stage of CKD and requires ongoing dialysis or a kidney transplant to survive. However, many patients suffer serious health consequences or die from CKD prior to developing ESRD. Currently, there is no cure for CKD and treatment involves management of the symptoms of the disease. Blood pressure medications, such as angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), are often prescribed to control hypertension, and hopefully, slow the progression of CKD. Nevertheless, according to the National Kidney Foundation, many of these patients continue to show declining kidney function. We believe DM199 offers a potentially novel approach for the treatment of CKD because KLK1 protein plays a vital role in normal kidney function. Since patients with moderate to severe CKD often excrete abnormally low levels of KLK1 in their urine, we believe that DM199 may prevent or reduce further kidney damage by increasing levels of KLK1 and restoring the protective KKS to regulate the production and release of nitric oxide and prostacyclin.
Acute Ischemic Stroke
According to the World Health Organization, each year approximately 15 million people worldwide suffer a stroke, of which 5.0 million will die and 5.0 million will be permanently disabled. According to the U.S. Center for Disease Control and Prevention approximately 87% of all strokes are ischemic in nature, a blockage of blood flow in/to the brain. We believe that stroke represents an area of significant unmet medical need and a KLK1 treatment (such as DM199) could provide a significant patient benefit, in particular given its proposed therapeutic window of up to 24 hours after the first sign of symptoms. Currently, the only FDA-approved pharmacological intervention for AIS is tissue plasminogen activator (tPA), which must be given within 4.5 hours of symptom onset. Treating patients with tPA during this time window can be challenging because it is difficult to determine precisely when symptoms began and a patient must undergo complex brain imaging before treatment to rule out a hemorrhagic stroke, a ruptured blood vessel causing bleeding within the brain. Mechanical thrombectomy, a procedure in which the clot is removed using catheter-based tools, is also available to certain patients. Despite the availability of these treatments, we believe they are relevant to approximately 10% of ischemic stroke patients due to the location of the clot, the elapsed time after the stroke occurred or other safety considerations. Thus, we believe DM199 may offer significant advantages over the current treatment options in that it fills a serious, unmet need for patients who cannot receive tPA or mechanical thrombectomy. Additionally, DM199 may also offer a complimentary follow-on treatment for patients who initially receive tPA or mechanical thrombectomy treatments by enabling sustained blood flow improvements to the brain during the critical weeks and months after a stroke. Based on the number of strokes each year (approximately 1.7 million in the U.S., Europe and Japan and 15 million worldwide) and considering the $8,500 estimated cost per patient for the current standard of care, tPA, we believe the annual market opportunity for DM199 could be significant.
Our Clinical Trials
Chronic Kidney Disease
In July 2019, we completed a Phase Ib clinical trial of DM199 in participants with moderate or severe CKD caused by Type I or Type II diabetes. We initiated dosing patients in this study in February 2019. The study was performed to assess the pharmacokinetics (PK) of three dose levels of DM199 (3, 5 and 8 µg/kg), administered in a single subcutaneous dose, as well as the evaluation of safety, tolerability and secondary pharmacodynamic (PD) endpoints. The study results demonstrated that at the 3µg/kg dose level, the PK profiles were similar between moderate and severe CKD patients, and consistent with healthy subjects (normal kidney function) tested previously. Additionally, DM199 was well tolerated with no dose-limiting tolerability observed. There were no deaths, no discontinuations due to a treatment-related adverse event (AE) and no treatment-related significant adverse events (SAEs). AEs were minor and consistent with standard treatment(s) in the CKD patient population. We announced favorable overall interim PD results from the first 28 subjects that included short-term improvements in Nitric Oxide (NO), average increase of 35.2%, Prostaglandin E2 (PGE2), average increase of 41.2%, estimated glomerular flow rate (eGFR), average increase of 4.08 mL/min/1732, and the urinary albumin to creatinine ratio (UACR) excluding subjects with normal UACR levels, average decrease of 18.7%. PD results appeared to be drug related in that the greatest improvements occurred approximately 24 hours after DM199 administration and subsequently declined.
In December 2019, we began enrolling patients in a Phase II CKD trial named REDUX, Latin for restore, a multi-center, open-label investigation of approximately 60 participants with CKD, who are being enrolled in two cohorts (30 per cohort). The study is being conducted in the United States at up to 10 sites and will be focused on participants with two specific causes of CKD. Cohort I of the study is focused on non-diabetic, hypertensive African Americans with Stage II or III CKD. African Americans are at greater risk for CKD than Caucasians, and those who have the APOL1 gene mutation are at an even higher risk. The study is designed to capture the APOL1 gene mutation as an exploratory biomarker in this cohort. Cohort II of the study is focused on participants with IgA Nephropathy (IgAN). The study will evaluate two dose levels of DM199 within each cohort. Study participants will receive DM199 by subcutaneous injection twice weekly for 95 days. The primary study endpoints include safety, tolerability, blood pressure, albuminuria and kidney function, which will be evaluated by changes from baseline in eGFR and albuminuria, as measured by the UACR.
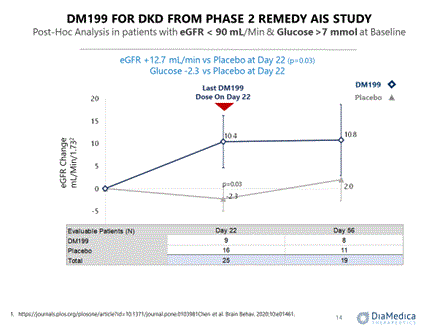
We intend to use a portion of the proceeds from this offering to begin enrollment in a third cohort in the REDUX trial comprised of participants with Type II diabetes mellitus with CKD, hypertension and albuminuria. In a post hoc analysis of endpoints in the ReMEDy trial, discussed below, a sub-set of participants with elevated blood glucose levels (>7 mmol/l) and impaired kidney function (eGFR <90) were observed to experience significant (mean +12.7 mL/min, p=0.03) improvement in kidney function as measured by the estimated glomerular filtration rate compared to placebo and a trending reduction in blood glucose levels (mean 2.2 mmol/l) compared to placebo. Initiation of this third cohort and development for this indication is contingent upon successful completion of this offering.
As of August 5, 2020, we have enrolled 18 subjects, including 7 African American subjects into cohort I and 11 subjects with IgAN into cohort II of the REDUX study. Due to actions implemented to combat the novel strain of the coronavirus (COVID-19) pandemic, we have experienced and continue to experience slower than expected enrollment in the REDUX clinical trial. We believe this is due to the reduction or suspension of activities at our clinical study sites as they address staff and patient safety concerns and patient concerns related to visiting clinical study sites in light of the pandemic. We anticipate that the COVID-19 pandemic will likely continue to adversely affect our ability to recruit or enroll subjects and we cannot provide any assurance as to when clinical sites will be able to resume enrollment at a normal rate or any guidance at this time as to when we will complete enrollment in the study. While results observed to date in the REDUX study indicate a safety profile consistent with past studies, there is insufficient data at this time to evaluate or comment upon efficacy.
Acute Ischemic Stroke
In May 2020, we announced top-line data from our Phase II ReMEDy trial assessing the safety, tolerability and markers of therapeutic efficacy of DM199 in patients suffering from AIS. We initiated treatment in this study in February 2018 and completed enrollment in October 2019 with 92 participants. The study drug (DM199 or placebo) was administered as an intravenous (IV) infusion within 24 hours of stroke symptom onset, followed by subcutaneous injections later that day and once every 3 days for 21 days. The study was designed to measure safety and tolerability along with multiple tests designed to investigate DM199’s therapeutic potential including plasma-based biomarkers and standard functional stroke measures assessed at 90 days post-stroke. Standard functional stroke measurements include the Modified Rankin Scale, National Institutes of Health Stroke Scale, the Barthel Index and C-reactive protein, a measure of inflammation. The study met primary safety and tolerability endpoints and there were no DM199-related serious adverse events. In addition, there was also a demonstrated therapeutic effect in participants that received tPA prior to enrollment but not in participants receiving mechanical thrombectomy prior to enrollment.
Prior to enrollment, 44 of the 91 evaluable patients (48%) received a mechanical thrombectomy, a catheter-based treatment indicated for those who have a large vessel occlusion and can be treated within 6 to 24 hours of the onset of stroke symptoms. While approximately 20% of AIS patients are believed to be eligible for a mechanical thrombectomy, currently only about 5% to 10% receive the treatment due to elapsed time post-stroke or unavailability of the therapy at the hospital where they present. DM199 is intended to treat the approximately 90% of AIS patients who do not receive either mechanical thrombectomy or tPA. Treatment for these patients is limited to palliative therapies. Due to the large volume of participants receiving mechanical thrombectomy prior to enrollment in ReMEDy, and a disproportionate distribution of these participants between the active treatment and placebo groups, DM199 did not produce a therapeutic effect in the overall study analysis.
When participants treated with mechanical thrombectomy are excluded from the study data set, representing the group of participants most closely aligned with the target treatment population for DM199, a positive therapeutic effect was demonstrated. As shown in the table below, when evaluating the participants treated with DM199 (n=25) vs. palliative therapies and/or tPA (n=21), the results showed that 36% of participants receiving DM199 progressed to a full or nearly full recovery at 90 days (NIHSS: 0-1), compared to 14% of participants in the placebo group. This represents a 22% absolute increase in the proportion of participants achieving a full or nearly full recovery. Additionally, subject deaths decreased from 24% in the placebo group to 12% in the active therapy group, a 50% relative reduction.
DM199 vs. Palliative Therapies and/or tPA
|
NIHSS Outcomes at 90 Days |
||||
|
0-1 |
2-8 |
≥ 9 |
Death |
|
|
Placebo (n=21) |
14% |
57% |
5% |
24% |
|
DM199 (n=24) |
36% |
36% |
16% |
12% |
In addition, in the evaluable participants (n=91), a significant reduction in the number of participants with severe recurrent stroke was noted in the active treatment group: 1 (2%) patient treated with DM199 vs. 7 (16%) on placebo (p=0.028), with 4 of the 7 resulting in participant death.
Further, in reviewing evaluable participants (n=91), improvements in the following biomarkers were observed in participants treated with DM199, which we believe are consistent with the DM199 mechanism of action:
|
● |
Increased NO (+105%) and PGE2 (+54%) were observed at day 22 vs baseline (p<0.05). Placebo group was not statistically significant vs baseline (p>0.05). These changes noted in the active treatment group did not reach statistical significance compared to placebo. |
|
● |
Reduction in C-reactive protein (CRP) of (-70%), a blood marker of inflammation, at 90 days. CRP decreased significantly vs. baseline (p<0.05), but was not statistically significant vs. placebo. The change in the placebo group was not statistically significant vs. baseline (p>0.05). |
|
● |
Reduction in elevated glucose levels in participants with type 2 diabetes, as defined by a blood glucose level >7 mmol/l (n=14), an average decrease of 1.9 mmol/l (p=0.06) in blood glucose levels of participants on active therapy was observed at day 22. In comparison, participants in the placebo group (n=16) showed an average increase of 0.08 mmol/l (p=0.94) at day 22. |
Changes in the eGFR, a measure of kidney function, were also analyzed in participants with eGFR <70 mL/Min/1.732 at baseline, which indicates the presence of CKD. Participants receiving DM199 exhibited a marked increase in eGFR at days 22 (last dose) and 56 (34 days post-treatment), as shown in the table below. eGFR at day 22 increased by at least 2 mL/Min in 77% of DM199 participants compared to 20% in placebo (p=0.007).
|
eGFR Mean Δ from Baseline (mL/Min/1.732) |
||
|
Day 22 (Last Dose) |
Day 56 (Off Treatment) |
|
|
Placebo |
+0.84 (n=15) |
-0.24 (n=12) |
|
DM199 |
+7.5 (n=13) |
+5.8 (n=12) |
|
Group Difference |
+6.6 |
+6.1 |
We believe these findings from our Phase II ReMEDy trial,which are consistent with Chinese data on the urine-derived form of KLK1, provide a signal that recombinant human KLK1 appears safe and may have promise as a new tool for physicians who have limited options for the treatment of patients suffering AIS and may also mitigate the adverse impact of ischemic stroke on kidney function.
Potential DM199 Commercial Advantages
The growing understanding of KLK1’s role in human health and its use in Asia as an approved therapeutic treatment highlights two important potential commercial advantages for DM199:
|
● |
KLK1 treatments currently sold in Japan, China and Korea. Research has shown that patients with low levels of KLK1 are associated with a variety of diseases related to vascular dysfunction, such as CKD, AIS, retinopathy and hypertension. Clinical trial data with human urine and porcine derived KLK1 has demonstrated statistically significant clinical benefits in treating a variety of patients with KLK1 compared to placebo. These efficacy results are further substantiated by established markets in Japan, China and Korea for pharmaceutical sales of KLK1 derived from human urine and porcine pancreas. We estimate that millions of patients have been treated with these forms of KLK1 in Asia. Altogether, we believe this supports a strong market opportunity for a synthetic version of KLK1 such as DM199. |
|
● |
KLK1 treatment has had limited side effects and has been well tolerated to date. KLK1 is naturally produced by the human body; and, therefore, the body’s own control mechanisms act to limit potential side effects. The only notable side effect observed in our clinical trials was orthostatic hypotension, or a sudden drop in blood pressure, which was only seen at doses ten to twenty times higher than our anticipated therapeutic dose levels. Moreover, routine clinical use of KLK1 treatment in Asia we understand has been well-tolerated by patients for several decades. In 2017, we completed a clinical trial comparing the pharmacokinetic profile of DM199 to the human urinary form of KLK1 (Kailikang), which showed DM199, when administered in intravenous form, had a similar pharmacokinetic profile. Further, when DM199 was administered subcutaneously, DM199 demonstrated a longer acting pharmacokinetic profile, superior to the intravenously administered Kailikang and DM199. |
In addition, we believe there are also significant formulation, manufacturing, regulatory and other advantages for our synthetic human KLK1 drug candidate DM199:
|
● |
Potency and Impurity Considerations. KLK1 derived from human urine or porcine pancreas may contain impurities, endotoxins, and chemical byproducts due to the inherent variability of the isolation and purification process. We believe that this creates the risk of inconsistencies in potency and impurities from one production run to the next. However, we expect to produce a consistent formulation of KLK1 that is free of endotoxins and other impurities. |
|
● |
Cost and Scalability. Large quantities of human urine and porcine pancreas must be obtained to derive a small amount of KLK1. This creates potential procurement, cost and logistical challenges to source the necessary raw material, particularly for human urine sourced KLK1. Once sourced, the raw material is processed using chemicals and costly capital equipment and produces a significant amount of byproduct waste. Our novel recombinant manufacturing process utilizes widely available raw materials and can be readily scaled for commercial production. Accordingly, we believe our manufacturing process will have significant cost and scalability advantages. |
|
● |
Regulatory. We are not aware of any attempts by manufacturers of the urine or porcine based KLK1 products to pursue regulatory approvals in the United States. We believe that this is related to challenges presented by using inconsistent and potentially hazardous biomaterials, such as human urine and porcine pancreas, and their resulting ability to produce a consistent drug product. Our novel recombinant manufacturing process utilizes widely available raw materials which we believe provides a significant regulatory advantage, particularly in regions such as the United States, Europe and Canada, where safety standards are high. In addition, we believe that DM199 could qualify for 12 years of data exclusivity under the Biologics Price Competition and Innovation Act of 2009, which was enacted as part of the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act of 2010. |
Our Strategy
We aim to become a leader in the discovery, development and commercialization of recombinant proteins for the treatment of severe and life-threatening diseases. To achieve this goal, we are pursuing the following strategies:
|
● |
Complete our ongoing Phase II studies for DM199 in CKD patients; |
|
● |
Commence a Phase III study for DM199 in AIS patients; |
|
● |
Explore potential new indications for DM199; and |
|
● |
Leverage our experience and technologies to develop new recombinant therapies and programs. |
Our Team
We have assembled a seasoned management team with extensive experience in drug discovery, development and manufacturing. Our Chief Executive Officer, Rick Pauls, MBA, is a successful venture capitalist and formerly the Co-Founder and Managing Director of CentreStone Ventures Inc., a life sciences venture capital fund which made early investments in DiaMedica. Our Chief Medical Officer, Harry Alcorn Jr., Pharm. D, has more than 30 years’ experience planning, operating, and executing clinical development programs across a range of diseases including kidney disease, diabetes, and cardiovascular disease, and most recently served as Chief Scientific Officer of DaVita Clinical Research. Our Vice President, Regulatory Affairs, Sydney Gilman, Ph.D., has more than 30 years’ experience in drug research, regulatory affairs and quality assurance, including six years as a chemistry reviewer in FDA’s Center for Drug Evaluation and Research. Edward Calamai, our consulting head of manufacturing, has over 30 years’ experience guiding manufacturing operations, including senior positions at Sensu and Seragen. Dr. Calamai is currently the Managing Partner at PM&C Associates, a company he co-founded in 2001. Our Chief Financial Officer, Scott Kellen, CPA, brings over two decades of operational and corporate finance expertise including an extensive background working with publicly-traded healthcare and biotechnology companies.
Risks Affecting Us
Please carefully consider the section titled “Risk Factors” beginning on page S-13 of this prospectus supplement, as well as risk factors referenced in the accompanying prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2019 and our Quarterly Report on Form 10-Q for the three months ended March 31, 2020, for a discussion of the factors you should carefully consider before deciding to purchase securities that may be offered by this prospectus supplement and the accompanying prospectus.
Additional risks and uncertainties not presently known to us may also impair our business operations. You should be able to bear a complete loss of your investment.
Financial Update
Our consolidated financial statements as of and for the three and six months ended June 30, 2020 are not expected to be available until after this offering is completed. Our preliminary research and development expense for the three and six months ended June 30, 2020 and cash, cash equivalents and marketable securities balance as of June 30, 2020 are set forth below. This financial information has been prepared by and is the responsibility of our management. Our independent registered public accounting firm has not audited, reviewed or performed any procedures with respect to this preliminary financial data or the accounting treatment thereof and does not express an opinion or any other form of assurance with respect thereto. We expect to announce our full financial results for the three and six months ended June 30, 2020 subsequent to the completion of this offering. While we are currently unaware of any items that would require us to make adjustments to the financial information set forth below, it is possible that we or our independent registered public accounting firm may identify such items as we finalize our unaudited financial statements, and any resulting changes could be material. Accordingly, undue reliance should not be placed on these preliminary estimates. These preliminary estimates are not necessarily indicative of any future period and should be read together with “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements,” and our consolidated financial statements and related notes incorporated by reference in this prospectus supplement and the accompanying prospectus.
|
(in thousands) |
Three Months Ended June 30, 2020 |
Six Months June 30, 2020 |
||||||
|
Research and development expense |
$ | 1,629 | $ | 3,010 | ||||
|
June 30, 2020 |
||||
|
Cash and cash equivalents |
4,955 | |||
|
Marketable securities |
6,844 | |||
|
Total cash and cash equivalents and marketable securities |
$ | 11,799 | ||
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion of revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act), and we may remain an emerging growth company for up to five years from December 31, 2018. However, if certain events occur prior to the end of such five-year period, including if we become a large accelerated filer, our annual gross revenue exceeds $1.07 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. In particular, we are required to only provide only two years of audited financial statements and are not required to disclose all of the executive compensation related information that would be required if we were not an emerging growth company. Accordingly, the information contained in our SEC reports may be different than the information you receive from other public companies in which you hold equity interests. However, we have irrevocably elected not to avail ourselves of the extended transition period for complying with new or revised accounting standards, and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Company Information
Our principal executive offices are located at Two Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447. Our telephone number is (763) 312-6755, and our Internet website address is www.diamedica.com. We make available on our website free of charge a link to our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as practicable after we electronically file such material with the SEC. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We are a corporation governed under British Columbia’s Business Corporations Act (BCBCA). Our company was initially incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of incorporation dated January 21, 2000. Our articles were amended (i) on February 26, 2001 to change our corporate name to DiaMedica Inc., (ii) on April 11, 2016 to continue the Company from The Corporations Act (Manitoba) to the Canada Business Corporations Act (CBCA), (iii) on December 28, 2016 to change our corporate name to DiaMedica Therapeutics Inc., (iv) on September 24, 2018 to permit us to hold shareholder meetings in the U.S. and to permit our directors, between annual general meetings of our shareholders, to appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of shareholders, (v) on November 15, 2018 to effect a 1-for-20 consolidation of our common shares, and (vi) on May 31, 2019, to continue our existence from a corporation incorporated under the CBCA into British Columbia under the BCBCA.
The Offering
|
Common shares offered by us |
shares. |
|
Option to purchase additional shares |
We have granted the underwriters an option for a period of up to 30 days from the date of this prospectus supplement to purchase up to an additional shares at the public offering price less the underwriting discounts and commissions. |
|
Common shares to be outstanding after this offering |
shares (or shares assuming the underwriters exercise in full their option to purchase additional shares). |
|
Use of proceeds |
We currently expect to use the net proceeds from this offering to continue our clinical and product development activities for DM199, including the addition of a new cohort III to our REDUX study to be comprised of participants with Type II diabetes mellitus with CKD, hypertension and albuminuria, and for other working capital and general corporate purposes. See “Use of Proceeds” on page S-20 of this prospectus supplement. |
|
Nasdaq Capital Market symbol |
“DMAC” |
|
Risk factors |
An investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page S-13 of this prospectus supplement, page 4 of the accompanying prospectus and in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus for a discussion of factors you should consider carefully when making an investment decision. |
The number of our common shares to be outstanding after this offering is based on 14,139,074 common shares outstanding as of March 31, 2020, and excludes as of that date the following:
|
● |
255,000 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $4.64 per share; |
|
● |
545,009 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.40 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
636,300 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,363,700 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
Except as otherwise indicated, all information in this prospectus supplement assumes no exercise by the underwriters of their option to purchase additional shares.
RISK FACTORS
Investing in our common shares involves a high degree of risk. Before investing in our common shares, you should carefully consider the risks described below, together with all of the other information contained in this prospectus supplement and the accompanying prospectus and incorporated by reference herein and therein, including from our Annual Report on Form 10-K for the year ended December 31, 2019 and our Quarterly Report on Form 10-Q for the period ended March 31, 2020, as well as any amendment or update to our risk factors reflected in subsequent filings with the SEC. Some of these factors relate principally to our business and the industry in which we operate. Other factors relate principally to your investment in our securities. The risks and uncertainties described therein and below are not the only risks facing us. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also materially and adversely affect our business and operations.
Risks Related to Our Business and Our Industry
The recent and ongoing COVID-19 pandemic could significantly disrupt our clinical trials and, therefore, our receipt of necessary regulatory approvals could be delayed or prevented.
The COVID-19 pandemic is having a severe effect on the clinical trials of many drug candidates. Some trials have been merely delayed, while others have been cancelled. The extent to which the COVID-19 pandemic may impact our ongoing and planned clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the duration and geographic reach of the outbreak, the severity of COVID-19, and the effectiveness of actions to contain and treat COVID-19. To date, the COVID-19 pandemic has caused significant delays in the enrollment of participants. The continued spread of COVID-19 could cause us to experience additional disruptions that could severely impact our business and clinical trials, including:
|
● |
additional delays or difficulties in enrolling and/or retaining participants in our clinical trials; |
|
● |
delays or difficulties in the initiation of additional clinical sites in the event that the current clinical sites are unable to recruit sufficient participants or at an acceptable rate; |
|
● |
delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials, including interruption in shipping that may affect the transport of clinical trial materials; |
|
● |
changes in local regulations as part of a response to the COVID-19 pandemic, which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether; |
|
● |
inability of participants to comply with clinical trial protocols, impede participant movement or interrupt healthcare services; |
|
● |
interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others, or interruption of clinical trial subject visits and study procedures, the occurrence of which could affect the integrity of clinical trial data; |
|
● |
risk that participants enrolled in our clinical trials will contract COVID-19 while the clinical trial is ongoing, which could result in participants dropping out of the trial, missing scheduled doses or follow-up visits or failing to follow protocol or otherwise impact the results of the clinical trial, including by increasing the number of observed adverse events; |
|
● |
delays in receiving authorizations from local regulatory authorities to initiate our planned clinical trials; |
|
● |
delays in necessary interactions with local regulatory authorities, ethics committees, and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; and |
|
● |
limitations in employee resources that would otherwise be focused on the conduct of our clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people. |
As a result, the expected timeline for data readouts of our clinical trials and certain regulatory filings may be negatively impacted, which would adversely affect our ability to initiate required phase III studies, obtain regulatory approval for and to commercialize our product candidates, increase our operating expenses and have a material adverse effect on our financial results.
We have conducted and may in the future conduct clinical trials for our product candidate outside the United States, and the FDA may not accept data from such trials.
We have conducted and may in the future conduct clinical trials for our product candidate outside the United States. For example, we conducted our ReMEDy Phase II clinical trial in Australia. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of such study data by the FDA is subject to certain conditions, and there can be no assurance that the FDA will accept data from the clinical trial we conducted in Australia or clinical trials we may conduct outside the United States in the future. For example, the clinical trial must be conducted in accordance with good clinical practices (GCP) requirements, and the FDA must be able to validate the data from the clinical trial through an onsite inspection if it deems such inspection necessary. In addition, when studies are conducted only at sites outside the United States, the FDA generally does not provide advance comment on the clinical protocols for the studies, and therefore there is an additional potential risk that the FDA could determine that the study design or protocol for a non-U.S. clinical trial was inadequate, which would likely require us to conduct additional clinical trials.
If the FDA does not accept data from the clinical trial we conducted in Australia, it would likely result in the need for additional clinical trials, which would be costly and time-consuming and delay aspects of our business plan, including the development and commercial launch of our DM199 product candidate. In addition, the conduct of clinical trials outside the United States also exposes us to additional risks, including risks associated with the following:
|
● |
foreign regulatory requirements that could burden or limit our ability to conduct our clinical trials; |
|
● |
administrative burdens of conducting clinical trials under multiple foreign regulatory schemes; |
|
● |
foreign exchange fluctuations; |
|
● |
compliance with foreign manufacturing, customs, shipment, and storage requirements; |
|
● |
cultural differences in medical practice and clinical research; and |
|
● |
diminished protection of intellectual property in some countries. |
Risks Related to Our Common Shares and this Offering
Our management will have broad discretion and flexibility as to how to use the net proceeds from this offering and may use the net proceeds in ways with which you disagree or which may not prove effective.
We currently intend to use the net proceeds from this offering as discussed under “Use of Proceeds” in this prospectus supplement. We have not allocated specific amounts of the net proceeds from this offering for any of the purposes set forth in that section. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
Purchasers of common shares in this offering will experience immediate and substantial dilution in the book value of their investment. You may experience further dilution upon exercise of our outstanding options and warrants.
The public offering price per common share in this offering is higher than the net tangible book value per common share before giving effect to this offering. Accordingly, if you purchase common shares in this offering, you will incur immediate substantial dilution of approximately $ per share, representing the difference between the public offering price of $ per common share, and our as adjusted net tangible book value per share as of March 31, 2020. In addition, if outstanding options or warrants are exercised, you could experience further dilution. For a further description of the dilution that you will experience immediately after this offering, see the section in this prospectus entitled “Dilution.”
Our common share price has been volatile and may continue to be volatile.
Our common shares trade on the Nasdaq Capital Market under the trading symbol “DMAC.” A number of factors could influence the volatility in the trading price of our common shares, including changes in the economy or in the financial markets, industry related developments, and the impact of material events and changes in our operations. Our quarterly losses may vary because of expenses we incur related to our R&D and clinical activities including the timing of costs for manufacturing DM199 and initiating and completing preclinical and clinical trials. Each of these factors could lead to increased volatility in the market price of our common shares. In addition, the market prices of the securities of our competitors may also lead to fluctuations in the trading price of our common shares. As a result of this volatility, you may not be able to sell your common shares at or above the public offering price.
We do not have a very active trading market for our common shares, and one may never develop.
Our common shares commenced trading in the United States on the Nasdaq Capital Market in December 2018. Previously our shares traded in Canada on the TSX Venture Exchange. We do not have a very active trading market for our common shares, and one may never develop, even after this offering. Although we anticipate a more active trading market for our shares will develop after this offering, we can give no assurance that this will occur or that an active trading market will be sustained following this offering. If an active market for our common shares does not develop, it may be difficult for you to sell our common shares you purchase in this offering at a favorable price or at all.
We may issue additional common shares resulting in share ownership dilution.
Future dilution will likely occur due to anticipated future equity issuances by us. To the extent we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted. In addition, as of March 31, 2020, we had outstanding warrants to purchase 255,000 common shares, options to purchase 1,181,309 common shares, deferred share units representing 21,183 common shares and 1,363,700 common shares reserved for future issuance in connection with future grants under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. If these or any future outstanding warrants, options, or deferred share units are exercised or otherwise converted into our common shares, our shareholders will experience additional dilution.
If there are substantial sales of our common shares or the perception that such sales could occur, the market price of our common shares could decline.
Sales of substantial numbers of our common shares or the perception that such sales could occur could cause a decline in the market price of our common shares. Any sales by existing shareholders or holders who exercise their warrants or stock options may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common shares.
We could be subject to securities class action litigation, which is expensive and could divert management attention.
In the past, securities class action litigation has often been brought against a company following a decline or increase in the market price of its securities or certain significant business transactions. We may become involved in this type of litigation in the future, especially if our clinical trial results are not successful or we enter into an agreement for a significant business transaction. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and our resources, which could harm our business. This is particularly true in light of our limited securities litigation insurance coverage.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, the market price of our common shares and trading volume could decline.
The market price and trading volume for our common shares in the United States after this offering will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will continue to cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our common shares or change their opinion of our common shares, the market price of our common shares would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the market price of our common shares or trading volume to decline.
We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to us as such may make our common shares less attractive to our shareholders and investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. We may remain an emerging growth company until December 31, 2023, the last day of the fiscal year following the fifth anniversary of our first sale of common shares pursuant to a registration statement under the Securities Act of 1933, as amended (the Securities Act) or until such earlier time as we have more than $1.07 billion in annual revenue, the market value of our common shares held by non-affiliates is more than $700 million or we issue more than $1 billion of non-convertible debt over a three-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (Section 404) not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Our shareholders and other investors may find our common shares less attractive as a result of our reliance on these exemptions. If some of our shareholders or other investors find our common shares less attractive as a result, there may be a less active trading market for our common shares, and the trading price of our common shares may be more volatile.
We are also a “smaller reporting company” under the federal securities laws and, as such, are subject to scaled disclosure requirements afforded to such companies. For example, as a smaller reporting company, we are subject to reduced executive compensation disclosure requirements.
Our shareholders and investors may find our common shares less attractive as a result of our status as an “emerging growth company” and “smaller reporting company” and our reliance on the reduced disclosure requirements afforded to these companies. If some of our shareholders or investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and the market price of our common shares may be more volatile.
Our inability to comply with Nasdaq’s continued listing requirements could result in our common shares being delisted, which could affect the market price and liquidity of our common shares and reduce our ability to raise capital.
We are required to meet certain qualitative and financial tests to maintain the listing of our common shares on the Nasdaq Capital Market. If we do not maintain compliance with Nasdaq’s continued listing requirements within specified periods and subject to permitted extensions, our common shares may be recommended for delisting (subject to any appeal we would file). No assurance can be provided that we will comply with these continued listing requirements. If our common shares were delisted, it could be more difficult to buy or sell our common shares and to obtain accurate quotations, and the price of our common shares could suffer a material decline. Delisting would also impair our ability to raise additional capital.
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the market price of our common shares.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders or other investors could lose confidence in our financial reporting, which would harm our business and could negatively impact the market price of our common shares. As a result of our limited administrative staffing levels, internal controls that rely on segregation of duties in many cases are not possible. Due to resource constraints and the present stage of our development, we do not have sufficient size and scale to warrant the hiring of additional staff to address this potential weakness at this time. To help mitigate the impact of this, we are highly reliant on the performance of compensating procedures and senior management’s review and approval. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles in the United States, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of shareholder or other investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the market price of our common shares.
Pursuant to Section 404, we are required to furnish a report by our management regarding our internal control over financial reporting, and if we become an accelerated filer under the federal securities laws, we will be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. There is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We have never paid dividends and do not expect to do so in the foreseeable future.
We have not declared or paid any cash dividends on our common shares to date. The payment of dividends in the future will be dependent on our earnings and financial condition and on such other factors as our Board of Directors considers appropriate. Unless and until we pay dividends, shareholders may not receive a return on their common shares. There is no present intention by our Board of Directors to pay dividends on our common shares. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, appreciation, if any, in the market price of our common shares will be your sole source of gain for the foreseeable future.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this prospectus supplement and the related prospectus supplement that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology, and the use of future dates. The forward-looking statements in or incorporated by reference into this prospectus supplement or the related prospectus supplement may include, among other things, statements about:
|
● |
our plans to develop, obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of CKD and AIS and our expectations regarding the benefits of our DM199 product candidate; |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain required regulatory approvals of our DM199 product candidate for CKD and AIS; |
|
● |
the perceived benefits of our DM199 product candidate over existing treatment options for CKD and AIS; |
|
● |
the potential size of the markets for our DM199 product candidate and our ability to serve those markets; |
|
● |
the rate and degree of market acceptance, both in the United States and internationally, of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for and commercialize our DM199 product candidate for CKD and AIS; |
|
● |
the success, cost and timing of planned clinical trials, as well as our reliance on collaboration with third parties to conduct our clinical trials; |
|
● |
our expectations regarding the impact of the COVID-19 pandemic on our business, including in particular the conduct of our clinical trials and the timing thereof; |
|
● |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
● |
expectations regarding federal, state, and foreign regulatory requirements and developments, such as potential FDA regulation of our DM199 product candidate for CKD and AIS; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain funding for our operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for our DM199 product candidate for CKD and AIS; |
|
● |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; and |
|
● |
our anticipated use of proceeds from this offering. |
Further, our preliminary financial information for the three and six months ended and as of June 30, 2020 are preliminary and subject to adjustment in the ongoing review procedures by our independent registered public accounting firm. In addition, any financial projections and other estimates contained herein are forward-looking statements with respect to our anticipated performance.
These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. In evaluating such forward-looking statements, you should specifically consider various factors that may cause actual results to differ materially from current expectations, including the risks and uncertainties outlined under the heading “Risk Factors” contained in this prospectus supplement and the accompanying prospectus, and in any other documents incorporated herein or therein (including in our most recent annual report on Form 10-K, subsequent quarterly reports on Form 10-Q and other filings we make with the SEC pursuant to Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended (the Exchange Act)).
The following are some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements: uncertainties with respect to: the possibility of unfavorable results from additional clinical trials of DM199 or from subsequent analysis of existing data from the ReMEDy study or existing or new data received from additional ongoing and future studies of DM199; the risk that existing preclinical and clinical data may not be predictive of the results of ongoing or later clinical trials; our plans to develop, obtain regulatory approval for and commercialize its DM199 product candidate for the treatment of CKD and AIS and its expectations regarding the benefits of DM199; our ability to conduct successful clinical testing of DM199 and within its anticipated parameters, costs and timeframes; the perceived benefits of DM199 over existing treatment options; the potential direct or indirect impact of the COVID-19 pandemic on our business; its reliance on collaboration with third parties to conduct clinical trials; its ability to continue to obtain funding for its operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for DM199 for CKD and AIS, and the risks identified under the heading “Risk Factors” contained in this prospectus supplement and the accompanying prospectus, and in any other documents incorporated herein or therein (including in our most recent annual report on Form 10-K, subsequent quarterly reports on Form 10-Q and other filings we make with the SEC pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act).
Any forward-looking statement in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, reflects our view, as at the respective dates of such documents, with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking statements. No forward-looking statement is a guarantee of future performance. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein contain estimates, projections and other information concerning our industry, our business and the markets for certain therapeutics, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of our common shares in this offering will be approximately $ million, or approximately $ million if the underwriters exercise their option to purchase additional shares in full, after deducting the estimated underwriting discounts and commissions and the estimated offering expenses payable by us.
We intend to use the net proceeds from this offering to continue our clinical and product development activities for DM199, including the addition of a new cohort III to our REDUX study to be comprised of participants with Type II diabetes mellitus with CKD, hypertension and albuminuria, and for other working capital and general corporate purposes.
The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing, progress and results of our clinical trials, the timing and progress of any partnering efforts, technological advances and the competitive environment for our products. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of our common shares offered by us hereunder. Accordingly, our management will have broad discretion in the timing and application of these proceeds.
Pending the uses described above, we may deposit the proceeds in our non-interest bearing checking account, interest bearing money market fund or invest them in short-term or marketable securities until we use them for their stated purpose.
DIVIDEND POLICY
We have not declared or paid any cash dividends on our common shares since our inception. We currently intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments, provisions of applicable law and other factors the Board deems relevant.
CAPITALIZATION
The following table sets forth our cash and capitalization as of March 31, 2020 (a) on an actual basis, and (b) on an as adjusted basis to give effect to the sale by us of common shares in this offering at the public offering price of $ per share, after deducting the underwriting discount and estimated offering expenses payable by us.
This table should be read with “Use of Proceeds” in this prospectus supplement, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included in our Quarterly Report on Form 10-Q for the three months ended March 31, 2020, which is incorporated by reference into this prospectus supplement.
|
As of March 31, 2020 |
||||||||
|
(unaudited) (in thousands) |
||||||||
|
Actual |
As Adjusted |
|||||||
|
Cash, cash equivalents and marketable securities |
$ | 12,648 | $ | |||||
|
Capitalization: |
||||||||
|
Shareholders’ equity: |
||||||||
|
Common shares, no par value; unlimited authorized; 14,139,074 actual issued and outstanding; as adjusted for this offering |
$ | – | $ | |||||
|
Additional paid-in capital |
72,323 | |||||||
|
Accumulated other comprehensive income |
42 | |||||||
|
Accumulated deficit |
(59,042 | ) | ||||||
|
Total shareholders’ equity |
$ | 13,323 | $ | |||||
|
Total capitalization |
$ | 14,365 | $ | |||||
The number of our common shares to be outstanding immediately after this offering, as shown above, is based on 14,139,074 common shares issued and outstanding as of March 31, 2020, and excludes as of that date:
|
● |
255,000 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $4.64 per share; |
|
● |
545,009 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.40 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
636,300 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,363,700 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
Except as otherwise indicated herein, all information in this prospectus supplement, including the number of shares that will be outstanding after this offering, does not assume or give effect to the exercise of the underwriters’ option to purchase additional shares in this offering.
DILUTION
If you invest in our common shares in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per common share and the as adjusted net tangible book value per share of our common shares immediately after this offering. Net tangible book value per common share is determined at any date by subtracting our total liabilities from the amount of our total tangible assets (total assets less intangible assets) and dividing the difference by the number of our common shares deemed to be outstanding at that date.
Our net tangible book value as of March 31, 2020 was approximately $13.3 million, or $0.94 per share, based on 14,139,074 common shares outstanding as of March 31, 2020. After giving effect to our sale of common shares in this offering at the public offering price of $ per share, after deducting estimated underwriting discounts and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2020 would have been $ million, or $ per share. This amount represents an immediate increase in net tangible book value of $ per common share to existing shareholders and an immediate dilution in net tangible book value of $ per common share to new investors purchasing common shares in this offering.
The following table illustrates this dilution on a per share basis:
|
Public offering price per share |
$ | |||||||
|
Net tangible book value per share as of March 31, 2020 |
$ | 0.94 | ||||||
|
Increase per share attributable to this offering |
$ | |||||||
|
Net tangible book value per share after this offering |
$ | |||||||
|
Dilution per share to new investors participating in this offering |
$ |
If the underwriters exercise their option in full to purchase additional common shares in this offering at the public offering price of $ per share, the as adjusted net tangible book value per share after the offering would be $ per share, the increase in the net tangible book value per share to existing stockholders would be $ per share and the dilution to purchasers in this offering would be $ per share.
The number of our common shares to be outstanding immediately after this offering, as shown above, is based on 14,139,074 common shares issued and outstanding as of March 31, 2020, and excludes as of that date:
|
● |
255,000 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $4.64 per share; |
|
● |
545,009 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.40 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
636,300 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.60 per share; and |
|
● |
1,363,700 common shares were reserved for future issuance in connection with future grants under DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
Except as otherwise indicated herein, all information in this prospectus supplement, including the number of shares that will be outstanding after this offering, does not assume or give effect to the exercise of the underwriters’option to purchase additional shares in this offering.
To the extent that options or warrants are exercised, other equity awards vest, new equity awards are issued under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan or pursuant to inducement awards, or we issue additional common shares in the future, there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing our common shares or other securities exercisable or exchangeable for, or convertible into, our common shares, your ownership will be further diluted.
DESCRIPTION OF COMMON SHARES
General
The following is a summary of the material terms of our common shares, as well as other material terms of our Notice of Articles and Articles and certain provisions of the BCBCA. References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value. This summary does not purport to be complete and is qualified in its entirety by the provisions of our Notice of Articles and Articles, which are included as exhibits to the registration statement of which this prospectus forms a part. For more information on how you can obtain our Notice of Articles and Articles, see the heading “Where You Can Find Additional Information.”
Authorized Share Capital
We have an authorized share capital consisting of an unlimited number of common shares, no par value per share.
Certain Rights of the Common Shares
Dividends
Holders of our common shares are entitled to share pro rata in such dividends as may be declared by our Board of Directors. Pursuant to the provisions of the BCBCA, we may not declare or pay a dividend if there are reasonable grounds for believing that we are, or would after the payment be, unable to pay our liabilities as they become due in the ordinary course of business. We may pay a dividend by issuing fully paid shares, bonds, debentures or other of our securities or in property (including money).
Liquidation, Dissolution or Winding-Up
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company or any other distribution of our assets among our shareholders for the purpose of winding-up our affairs, holders of common shares are entitled to share pro rata in our assets available for distribution after we pay our creditors.
Voting Rights and Shareholders’ Meetings
Holders of our common shares are entitled to receive notice of and to attend and vote at all meetings of our shareholders. Each holder of our common shares is entitled to one vote, either in person or by proxy, on all matters submitted to shareholders.
Our Board of Directors must call an annual general meeting of shareholders to be held not later than 15 months after the last preceding annual general meeting of shareholders but no later than six months after the end of our preceding financial year-end and may, at any time, call a special meeting of shareholders. Under our Articles, a meeting of our shareholders may be held anywhere in or outside of British Columbia, as determined by the Board of Directors. For purposes of determining the shareholders who are entitled to receive notice of or to vote at a meeting of shareholders, the Board of Directors may, in accordance with National Instrument 54-101 - Communications with Beneficial Owners of Securities of a Reporting Issuer of the Canadian Securities Administrators, fix in advance a date as the record date for that determination of shareholders, but that record date may not be more than 60 days or less than 30 days before the date on which the meeting is to be held.
Our Articles provide that notice of the time and place of a meeting of shareholders must be sent to each shareholder entitled to vote at the meeting, each director and to our auditors, not more than 50 days and not less than 21 days prior to the meeting. Under our Articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding not less than one-third (33 1/3) of the issued common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting. A shareholder may participate in a meeting by means of telephone or other communication medium that permits all persons participating in the meeting to communicate with each other during the meeting.
In the case of joint shareholders, one of the holders present at a meeting, either personally or by proxy, may, in the absence of the other holder(s) of the shares, vote the shares. If two or more joint shareholders are present, personally or by proxy, then only the vote of the joint shareholder present whose name stands first on the central securities register in respect of the share will be counted.
No Preemption Rights; Limited Restrictions on Directors’ Authority to Issue Common Shares
Existing holders of our common shares have no rights of preemption or first refusal under our Articles or the BCBCA with respect to future issuances of our common shares. The common shares do not have conversion rights, are not subject to redemption and do not have the benefit of any sinking fund provisions. Subject to the rules and policies of the Nasdaq Stock Market and applicable corporate and securities laws, our Board of Directors has the authority to issue additional common shares.
Amendments to Articles
The Articles and the BCBCA govern the rights of holders of our common shares.
Subject to the BCBCA, unless an alteration to the Company’s Notice of Articles would be required, our directors can authorize the alteration of our Articles to, among other things, create additional classes or series of shares or, if none of the shares of a class or series are allotted or issued, eliminate that class or series of shares.
Subject to the BCBCA, our shareholders can authorize the alteration of our Articles and Notice of Articles to create or vary the rights or restrictions attached to any class of our shares by passing an ordinary resolution at a duly convened meeting of shareholders. An alteration to the Company’s Notice of Articles will not be effective until the notice of alteration is filed with the registrar pursuant to the BCBCA. An alteration to the Company’s Articles, which is not an alteration to the Company’s Notice of Articles, will be effective on the date and time that the resolution is received for deposit at the Company’s records office.
Fundamental Changes
Pursuant to the BCBCA, we may not effect any of the following fundamental changes without the consent of the holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and separately as a class at a duly convened meeting of our shareholders:
|
● |
any proposed amalgamation (consolidation or merger) involving our company in respect of which the BCBCA requires that the approval of our shareholders be obtained; |
|
● |
any proposed plan of arrangement pursuant to the BCBCA involving our company in respect of which the BCBCA or any order issued by an applicable court requires that the approval of our shareholders be obtained; |
|
● |
any proposed sale, lease or exchange of all or substantially all of our undertaking; and |
|
● |
any voluntary liquidation of our company. |
Election and Removal of Directors
At each annual general meeting of shareholders, our shareholders are required to elect directors to hold office for a term expiring not later than the close of the next annual general meeting of shareholders. Our Board of Directors may fill vacancies among the Board. Our directors may also, between annual general meetings of our shareholders, appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third (1/3) of the number of directors who held office at the expiration of the last meeting of shareholders.
Since shareholders do not have cumulative voting rights, holders of more than 50% of our outstanding common shares can elect all of our directors if they choose to do so. In such event, holders of the remaining shares will be unable to elect any director.
Under the BCBCA, a public company must have a minimum of three directors, who are not required to be resident Canadians.
Under the BCBCA, a director may be removed by shareholders by special resolution unless the Articles provide for a lower approval level. The Articles allow shareholders to remove directors by a special resolution if approved by holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and voting separately as a class at a duly convened meeting of our shareholders.
Registration Rights
We have not granted any rights to have our common shares or other securities registered under the Securities Act.
Listing
Our common shares are listed and trade in the United States on the Nasdaq Capital Market under the trading symbol “DMAC.”
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
Limitation of Liability and Indemnification Matters
Our Articles provide that we will indemnify our directors, former directors, his or her heirs and legal personal representatives and other individuals as we may determine against all eligible penalties to which such person is or may be liable to the fullest extent permitted by British Columbia law. We will pay all expenses actually and reasonably incurred by such person, either as such expenses are incurred in advance of the final disposition of an eligible proceeding or after the final disposition of an eligible proceeding. British Columbia law provides that a company must not indemnify its directors if any of the following circumstances apply:
|
● |
if the indemnity or payment is made under an earlier agreement to indemnify or pay expenses and, at the time that the agreement to indemnify or pay expenses was made, the company was prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if the indemnity or payment is made otherwise than under an earlier agreement to indemnify or pay expenses and, at the time that the indemnity or payment is made, the company is prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if, in relation to the subject matter of the relevant proceeding, the director did not act honestly and in good faith with a view to the best interests of the company or the associated corporation, as the case may be, with such associated corporation being an affiliate of the company or a partnership, trust, joint venture or other unincorporated entity in which the director served in the capacity as a director or a position equivalent to that thereof, at the request of the company; or |
|
● |
in the case of the relevant proceeding other than a civil proceeding, if the director did not have reasonable grounds for believing that the director’s conduct in respect of which the proceeding was brought was lawful. |
Notwithstanding any of the above prohibitions, the company or a director may apply to court for an order that the company must indemnify the director for any liability or expenses incurred by the director or for any other related obligations of the company.
The Articles also permit us to purchase insurance on behalf of any officer, director, employee or other agent of our company, of an affiliated entity, or, at our request, of another entity, for any liability arising out of that person’s actions in such capacity. We have entered into indemnification agreements with each of our current directors and executive officers requiring us to indemnify these individuals to the fullest extent permitted under British Columbia law against liability that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified, and have received a written undertaking from each such director and officer as required under British Columbia law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Takeover Laws
All provinces of Canada have adopted National Instrument 62-104 entitled “Take-Over Bids and Issuer Bids” and related forms to harmonize and consolidate take-over bid and issuer bid regimes nationally (NI 62-104). The Canadian Securities Administrators, or CSA, have also issued National Policy 62-203 entitled “Take-Over Bids and Issuer Bids” (the National Policy) which contains regulatory guidance on the interpretation and application of NI 62-104 and on the conduct of parties involved in a bid. The National Policy and NI 62-104 are collectively referred to as the “Bid Regime.” The National Policy does not have the force of law, but is an indication by the CSA of what the intentions and desires of the regulators are in the areas covered by their policies. Unlike some regimes where the take-over bid rules are primarily policy-driven, in Canada the regulatory framework for take-over bids is primarily rules-based, which rules are supported by policy.
A “take-over bid” or “bid” is an offer to acquire outstanding voting or equity securities of a class made to any person who is in one of the provinces of Canada or to any securityholder of an offeree issuer whose last address as shown on the books of a target is in such province, where the securities subject to the offer to acquire, together with the securities “beneficially owned” by the offeror, or any other person acting jointly or in concert with the offeror, constitute in the aggregate 20% or more of the outstanding securities of that class of securities at the date of the offer to acquire. For the purposes of the Bid Regime, a security is deemed to be “beneficially owned” by an offeror as of a specific date if the offeror is the beneficial owner of a security convertible into the security within 60 days following that date, or has a right or obligation permitting or requiring the offeror, whether or not on conditions, to acquire beneficial ownership of the security within 60 days by a single transaction or a series of linked transactions. Offerors are also subject to early warning requirements, where an offeror who acquires “beneficial ownership of”, or control or direction over, voting or equity securities of any class of a reporting issuer or securities convertible into, voting or equity securities of any class of a target that, together with the offeror’s securities, would constitute 10% or more of the outstanding securities of that class must promptly publicly issue and file a news release containing certain prescribed information, and, within two business days, file an early warning report containing substantially the same information as is contained in the news release
In addition, where an offeror is required to file an early warning report or a further report as described and the offeror acquires or disposes of beneficial ownership of, or the power to exercise control or direction over, an additional 2% or more of the outstanding securities of the class, or disposes of beneficial ownership of outstanding securities of the class below 10%, the offeror must issue an additional press release and file a new early warning report. Any material change in a previously filed early warning report also triggers the issuance and filing of a new press release and early warning report. During the period commencing on the occurrence of an event in respect of which an early warning report is required and terminating on the expiry of one business day from the date that the early warning report is filed, the offeror may not acquire or offer to acquire beneficial ownership of any securities of the class in respect of which the early warning report was required to be filed or any securities convertible into securities of that class. This requirement does not apply to an offeror that has beneficial ownership of, or control or direction over, securities that comprise 20% of more of the outstanding securities of the class.
Related party transactions, issuer bids and insider bids are subject to additional regulation that may differ depending on the particular jurisdiction of Canada in which it occurs.
In addition to the foregoing, certain other Canadian legislation may limit a Canadian or non-Canadian entity’s ability to acquire control over or a significant interest in us, including the Competition Act (Canada) and the Investment Canada Act (Canada). Issuers may also approve and adopt shareholder rights plans or other defensive tactics designed to be triggered upon the commencement of an unsolicited bid and make the company a less desirable takeover target.
Other Canadian Laws Affecting U.S. Shareholders
There are no governmental laws, decrees or regulations in Canada relating to restrictions on the export or import of capital, or affecting the remittance of interest, dividends or other payments by us to our shareholders who are non-residents of Canada, other than Canadian withholding tax as discussed below.
Dividends paid or credited (or deemed to be paid or credited) by the Company to residents of the United States of America within the meaning of the Canada-United States Tax Convention (1980), as amended (US Treaty) are generally subject to a 15% withholding tax on the amount of the dividends (see discussion below under “Material Canadian Federal Income Tax Considerations—Dividends).
There are no limitations specific to the rights of non-residents of Canada to hold or vote our common shares under the BCBCA, or in our Notice of Articles or Articles, other than those imposed by the Investment Canada Act (Canada) as discussed below.
Non-Canadian investors who acquire a controlling interest in us may be subject to the Investment Canada Act (Canada), which governs the basis on which non-Canadians may invest in Canadian businesses. Under the Investment Canada Act (Canada), the acquisition of a majority of the voting interests of an entity (or of a majority of the undivided ownership interests in the voting common shares of an entity that is a corporation) is deemed to be an acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of the voting common shares. The acquisition of less than one-third of the voting common shares of a corporation (or of an equivalent undivided ownership interest in the voting common shares of the corporation) is deemed not to be acquisition of control of that corporation.
Differences in Corporate Law
We are governed by the BCBCA, which is generally similar to laws applicable to United States corporations. Significant differences between the BCBCA and the Delaware General Corporate Law (DGCL), which governs companies incorporated in the State of Delaware, include the following:
|
Capital Structure |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the certificate of incorporation must set forth the total number of shares of stock which the corporation shall have authority to issue and the par value of each of such shares, or a statement that the shares are to be without par value. |
Under the BCBCA, the notice of articles of a corporation must describe the authorized share structure of the corporation. |
|
|
Dividends |
||
|
Delaware |
British Columbia |
|
|
The DGCL generally provides that, subject to certain restrictions, the directors of a corporation may declare and pay dividends upon the shares of its capital stock either out of the corporation’s surplus or, if there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. Further, the holders of preferred or special stock of any class or series may be entitled to receive dividends at such rates, on such conditions and at such times as stated in the certificate of incorporation. |
Under the BCBCA, dividends may be declared on the common shares at the discretion of the board of directors. Any dividends declared shall be subject to the rights, if any, of shareholders holding shares with special rights as to dividends.
Our directors may declare dividends unless there are reasonable grounds for believing that the corporation is insolvent or the payment of such dividends would render the company insolvent. |
|
Number and Election of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the board of directors must consist of at least one person, and the number of directors is generally fixed by, or in the manner provided in, the bylaws of the corporation, unless the certificate of incorporation fixes the number of directors, in which case a change in the number of directors shall be made only by amendment of the certificate.
The Board may be divided into three classes of directors, with one-third of each class subject to election by the stockholder each year after such classification becomes effective. |
Pursuant to the BCBCA, a public company must have at least three directors.
In accordance with our Articles, all directors cease to hold office immediately before the election or appointment of directors at every annual general meeting of shareholders, but are eligible for re-election or re-appointment. |
|
|
Removal of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, any or all directors may be removed with or without cause by the holders of a majority of shares entitled to vote at an election of directors unless the certificate of incorporation otherwise provides or in certain other circumstances if the corporation has cumulative voting. |
As permitted under the BCBCA, our Articles provide that a director may be removed before the expiration of their term by a special resolution of shareholders. Our Articles also provide that the directors may remove any director before the expiration of their term if the director is charged with an indictable offence or if the director ceases to be qualified to act as a director and does not promptly resign, and the directors may appoint a director to fill the resulting vacancy. |
|
|
Vacancies on the Board of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, vacancies and newly created directorships resulting from an increase in the authorized number of directors may be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director. |
Under the BCBCA, casual vacancies on the board may be filled by the remaining directors. If a vacancy on the board occurs as a result of the removal of a director, the vacancy may be filled by the shareholders at the shareholders meeting, if any, at which the director is removed, or if not filled in that manner, by the shareholders or the remaining directors. |
|
|
Qualifications of Directors |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, directors are required to be natural persons, but are not required to be residents of Delaware. The certificate of incorporation or bylaws may prescribe other qualifications for directors. |
Under the BCBCA, directors are not required to be residents of British Columbia. The articles of a corporation may prescribe other qualifications for directors. |
|
Board of Director Quorum and Vote Requirements |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a majority of the total number of directors shall constitute a quorum for the transaction of business unless the certificate or bylaws require a greater number. The bylaws may lower the number required for a quorum to one-third the number of directors, but no less.
Under the DGCL, the board of directors may take action by the majority vote of the directors present at a meeting at which a quorum is present unless the certificate of incorporation or bylaws require a greater vote. |
Under the BCBCA, a majority of the number of directors or minimum number of directors required by the articles constitutes a quorum at any meeting. |
|
|
Transactions with Directors and Officers |
||
|
Delaware |
British Columbia |
|
|
The DGCL generally provides that no transaction between a corporation and one or more of its directors or officers, or between a corporation and any other corporation or other organization in which one or more of its directors or officers, are directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the board or committee which authorizes the transaction, or solely because any such director’s or officer’s votes are counted for such purpose, if: (i) the material facts as to the director’s or officer’s interest and as to the transaction are known to the board of directors or the committee, and the board or committee in good faith authorizes the transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; (ii) the material facts as to the director’s or officer’s interest and as to the transaction are disclosed or are known to the stockholders entitled to vote thereon, and the transaction is specifically approved in good faith by vote of the stockholders; or (iii) the transaction is fair as to the corporation as of the time it is authorized, approved or ratified, by the board of directors, a committee or the stockholders. |
Under the BCBCA, a director or senior officer who holds a disclosable interest in a material contract or transaction into which a corporation has entered or proposes to enter may generally not vote on any directors’ resolution to approve the contract or transaction. A director or senior officer has a disclosable interest in a material contract or transaction if (a) the contract or transaction is material to the corporation, (b) the corporation has entered, or proposes to enter, into the contract or transaction, and (c) either of the following applies to the director or senior officer: (i) the director or senior officer has a material interest in the contract or transaction, or (ii) the director or senior officer is a director or senior officer of, or has a material interest in, a person who has a material interest in the contract or transaction.
Under the BCBCA, directors or senior officers do not have a disclosable interest in a contract or transaction merely because the contract or transaction relates to the remuneration of the director or senior officer in that person’s capacity as director, officer, employee or agent of the corporation or of an affiliate of the corporation. |
|
|
Limitation on Liability of Directors |
||
|
Delaware |
British Columbia |
|
|
The DGCL permits a corporation to include a provision in its certificate of incorporation eliminating or limiting the personal liability of a director to the corporation or its stockholders for monetary damages for a breach of the director’s fiduciary duty as a director, except: ● for breach of the director’s duty of loyalty to the corporation or its stockholders; ● for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of the law; ● under Section 174 of the DGCL, which concerns unlawful payment of dividends, stock purchases or redemptions; or ● for any transaction from which the director derived an improper personal benefit. |
No provision in a contract or the articles relieves a director or officer from the duty to act in accordance with the BCBCA and the regulations, or relieves them from liability for a breach thereof. |
|
Indemnification of Directors and Officers |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a corporation may indemnify any person who is made a party to any third-party action, suit or proceeding on account of being a director, officer, employee or agent of the corporation (or was serving at the request of the corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise) against expenses, including attorney’s fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with the action, suit or proceeding through, among other things, a majority vote of a quorum consisting of directors who were not parties to the suit or proceeding, if the person:
● acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation; ● in some circumstances, acted at least not opposed to its best interests; and ● in a criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL permits indemnification for derivative suits against expenses (including legal fees) if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and only if the person is not found liable, unless a court determines the person is fairly and reasonably entitled to the indemnification. |
Under the BCBCA, a corporation may indemnify a director or officer of the corporation, a former director or officer of the corporation, or another individual who acts or acted at the corporation’s request as a director or officer, or an individual acting in a similar capacity, of another entity (an “eligible party”), against all judgments, penalties or fines awarded or imposed in, or an amount paid in settlement of (an “eligible penalty”) a proceeding in which the eligible party or any of the heirs and personal representatives of the eligible party, by reason of the eligible party being or having been a director or officer of, or holding or having held a position equivalent to that of a director or officer, the corporation or an associated corporation is or may be joined as a party, or is or may be liable for or in respect of a judgment, penalty or fine in, or expenses related to, the proceeding (an “eligible proceeding”).
Under the BCBCA, a corporation must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by the eligible party in respect of that proceeding if the eligible party has not been reimbursed for those expenses and is wholly successful, on the merits or otherwise, in the outcome of the proceeding or is substantially successful on the merits in the outcome of the proceeding.
Under the BCBCA, a corporation may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an eligible party in respect of that proceeding. Notwithstanding the foregoing, a corporation must not make any such payments unless the corporation first receives from the eligible party a written undertaking that, if it is ultimately determined that the payment of the expenses is prohibited under the BCBCA, the eligible party will repay the amounts advanced.
A corporation may not indemnify an eligible party or pay the expenses of an eligible party: ● if, in relation to the subject matter of the eligible proceeding, the eligible party did not act honestly and in good faith with a view to the best interests of the corporation or the associated corporation, as the case may be; |
|
● in the case of an eligible proceeding other than a civil proceeding, if the eligible party did not have reasonable grounds for believing that the eligible party’s conduct in respect of which the proceeding was brought was lawful.
If an eligible proceeding is brought against an eligible party by or on behalf of the corporation or by or on behalf of an associated corporation, the corporation must not indemnify an eligible party in respect of the proceeding or pay the expenses of the eligible party in respect of the proceeding. |
||
|
Call and Notice of Shareholder Meetings |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, an annual or special stockholder meeting is held on such date, at such time and at such place as may be designated by the board of directors or any other person authorized to call such meeting under the corporation’s certificate of incorporation or bylaws.
If an annual meeting for election of directors is not held on the date designated or an action by written consent to elect directors in lieu of an annual meeting has not been taken within 30 days after the date designated for the annual meeting, or if no date has been designated, for a period of 13 months after the later of the last annual meeting or the last action by written consent to elect directors in lieu of an annual meeting, the Delaware Court of Chancery may summarily order a meeting to be held upon the application of any stockholder or director.
Special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
Under the BCBCA, the directors are required to call an annual meeting of shareholders not later than 18 months after the date the corporation was recognized, and subsequently, at least once in each calendar year and not more than 15 months after the last annual reference date.
As permitted by the BCBCA, our Articles stipulate that a meeting of our shareholders may be held in or outside of British Columbia as determined by the board of directors.
The directors may at any time call a special meeting of the shareholders. The holders of not less than five per cent of the issued shares of a corporation that carry the right to vote at a meeting may requisition the directors to call a meeting of shareholders for the purposes stated in the requisition. |
|
|
Shareholder Action by Written Consent |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a majority of the stockholders of a corporation may act by written consent without a meeting unless such action is prohibited by the corporation’s certificate of incorporation. |
Under the BCBCA, shareholders may act by written resolution signed by all the shareholders entitled to vote on that resolution at a meeting of shareholders. |
|
Shareholder Nominations and Proposals |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, the bylaws of a corporation may include provisions respecting the nomination of directors or proposals by stockholders, including requirements for advance notice to the corporation. |
Subject to the BCBCA, a registered owner or beneficial owner of one or more shares that carry the right to vote at general meetings and who has been a registered owner or beneficial owner of one or more such shares for an uninterrupted period of at least 2 years may submit to the corporation a proposal of a matter that the person wishes to have considered at the next annual general meeting of the corporation. Any such proposal must, among other things, be supported by qualified shareholders who constitute at least 1/100 of the issued common shares of the company that carry the right to vote at general meetings, or have a fair market value in excess of CDN$2,000. |
|
|
Shareholder Quorum and Vote Requirements |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, quorum for a stock corporation is a majority of the shares entitled to vote at the meeting unless the certificate of incorporation or bylaws specify a different quorum, but in no event may a quorum be less than one-third of the shares entitled to vote. Unless the DGCL, certificate of incorporation or bylaws provide for a greater vote, generally the required vote under the DGCL is a majority of the shares present in person or represented by proxy, except for the election of directors which requires a plurality of the votes cast. |
Unless the articles otherwise provide, under the BCBCA a quorum of shareholders is present at a meeting of shareholders, irrespective of the number of persons actually present at the meeting, if the holders of a majority of the shares entitled to vote at the meeting are present in person or represented by proxy. Under our articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding, in the aggregate, not less than 33 1/3% of the outstanding voting common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting.
Unless the BCBCA or articles provide for a greater vote, generally the required vote under the BCBCA is by ordinary resolution, or a resolution passed by a majority of the votes cast by the shareholders who voted in respect of that resolution. |
|
|
Amendment of Governing Instrument |
||
|
Delaware |
British Columbia |
|
|
Amendment of Certificate of Incorporation. Generally, under the DGCL, the affirmative vote of the holders of a majority of the outstanding stock entitled to vote is required to approve a proposed amendment to the certificate of incorporation, following the adoption of the amendment by the board of directors of the corporation, provided that the certificate of incorporation may provide for a greater vote. Under the DGCL, holders of outstanding shares of a class or series are entitled to vote separately on an amendment to the certificate of incorporation if the amendment would have certain consequences, including changes that adversely affect the rights and preferences of such class or series.
Amendment of Bylaws. Under the DGCL, after a corporation has received any payment for any of its stock, the power to adopt, amend or repeal bylaws shall be vested in the stockholders entitled to vote; provided, however, that any corporation may, in its certificate of incorporation, provide that bylaws may be adopted, amended or repealed by the board of directors. The fact that such power has been conferred upon the board of directors shall not divest the stockholders of the power nor limit their power to adopt, amend or repeal the bylaws. |
Amendment to Notice of Articles. Under the BCBCA, an amendment to a corporation’s notice of articles generally requires a special resolution of shareholders. A special resolution is a resolution passed by a majority of not less than two-thirds of the votes cast by the shareholders who voted in respect of the resolution or signed by all shareholders entitled to vote on that resolution.
Amendment of Articles. Unless the articles otherwise provide, the directors may, by resolution, make, amend or repeal any articles that regulate the business or affairs of the corporation. |
|
Votes on Mergers, Consolidations and Sales of Assets |
||
|
Delaware |
British Columbia |
|
|
The DGCL provides that, unless otherwise provided in the certificate of incorporation or bylaws, the adoption of a merger agreement requires the approval of a majority of the outstanding stock of the corporation entitled to vote thereon. |
Under the BCBCA, the approval of an amalgamation agreement requires approval by special resolution. |
|
|
Dissenters’ Rights of Appraisal |
||
|
Delaware |
British Columbia |
|
|
Under the DGCL, a stockholder of a Delaware corporation generally has the right to dissent from and request payment for the stockholders shares upon a merger or consolidation in which the Delaware corporation is participating, subject to specified procedural requirements, including that such dissenting stockholder does not vote in favor of the merger or consolidation. However, the DGCL does not confer appraisal rights, in certain circumstances, including if the dissenting stockholder owns shares traded on a national securities exchange and will receive publicly traded shares in the merger or consolidation. Under the DGCL, a stockholder asserting appraisal rights does not receive any payment for his or her shares until the court determines the fair value or the parties otherwise agree to a value. The costs of the proceeding may be determined by the court and assessed against the parties as the court deems equitable under the circumstances. |
Under the BCBCA, a shareholder may dissent from a transaction when the corporation resolves to: (a) amend its articles to alter a restriction on the powers of the corporation or on the business the corporation is permitted to carry on; (b) adopt an amalgamation agreement; (c) to approve an arrangement, the terms of which arrangement permit dissent; (d) authorize or ratify the sale, lease or other disposition of all or substantially all of the corporation’s undertaking; (e) be continued under the laws of another jurisdiction.
A shareholder asserting dissenters rights is entitled, subject to specified procedural requirements, including objecting to the action giving rise to dissenters rights and making a proper demand for payment, to be paid by the corporation the fair value of the shares in respect of which the shareholder dissents. Under the BCBCA, if the shareholder and the corporation do not agree on the fair value for the shareholders shares, the corporation or the dissenting shareholder may apply to a court to fix a fair value for the shares. |
|
|
Anti-takeover and Ownership Provisions |
||
|
Delaware |
British Columbia |
|
|
Unless an issuer opts out of the provisions of Section 203 of the DGCL, Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with a holder of 15% or more of the corporation’s voting stock (as defined in Section 203), referred to as an interested stockholder, for a period of three years after the date of the transaction in which the interested stockholder became an interested stockholder, except as otherwise provided in Section 203. For these purposes, the term “business combination” includes mergers, assets sales and other similar transactions with an interested stockholder. |
The BCBCA contains no restriction on adoption of a shareholder rights plan. The BCBCA does not restrict related party transactions; however, in Canada, takeovers and other related party transactions are addressed in provincial securities legislation and policies. |
CERTAIN UNITED STATES INCOME TAX CONSIDERATIONS
The following discussion is generally limited to certain material U.S. federal income tax considerations relating to the purchase, ownership and disposition of our common shares by U.S. Holders (as defined below). This discussion applies to U.S. Holders that hold our common shares as capital assets. This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of our common shares. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Although this discussion is generally limited to the U.S. federal income tax considerations to U.S. Holders, the U.S. federal income tax treatment of dividends on and gain on sale or exchange of our common shares by certain “Non-U.S. Holders” (as defined below) is included below at “U.S. Federal Income Taxation of Non-U.S. Holders.”
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (IRS) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions presented in this summary. In addition, because the guidance on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions described in this summary.
This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (Code), U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, and the income tax treaty between the United States and Canada (Convention), all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect. This summary is applicable to U.S. Holders who are residents of the United States for purposes of the Convention and who qualify for the full benefits of the Convention. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
This discussion does not address all of the U.S. federal income tax considerations that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents of the United States, persons who hold common shares as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons that have a “functional currency” other than the U.S. dollar, persons that own (or are deemed to own) 10% or more (by voting power or value) of our common shares, persons that acquire their common shares as part of a compensation arrangement, corporations that accumulate earnings to avoid U.S. federal income tax, partnerships and other pass-through entities, and investors in such pass-through entities). This discussion does not address any U.S. state or local or non-U.S. tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements.
As used in this discussion, the term “U.S. Holder” means a beneficial owner of common shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
If an entity treated as a partnership for U.S. federal income tax purposes holds the common shares, the U.S. federal income tax considerations relating to an investment in the common shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor regarding the U.S. federal income tax considerations applicable to it and its partners of the purchase, ownership and disposition of the common shares.
Persons holding common shares should consult their own tax advisors as to the particular tax considerations applicable to them relating to the purchase, ownership and disposition of common shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Distributions
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” a U.S. Holder that receives a distribution with respect to the common shares generally will be required to include the gross amount of such distribution (before reduction for any Canadian withholding taxes) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s common shares. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s common shares, the remainder will be taxed as capital gain. However, we cannot provide any assurance that we will maintain or provide earnings and profits determinations in accordance with U.S. federal income tax principles. Therefore, U.S. Holders should expect that a distribution will generally be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
The U.S. dollar value of any distribution on the common shares made in Canadian dollars generally should be calculated by reference to the exchange rate between the U.S. dollar and the Canadian dollar in effect on the date of receipt (or deemed receipt) of such distribution by the U.S. Holder regardless of whether the Canadian dollars so received are in fact converted into U.S. dollars at that time. If the Canadian dollars received are converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally should not recognize currency gain or loss on such conversion. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally will have a basis in such Canadian dollars equal to the U.S. dollar value of such Canadian dollars on the date of receipt (or deemed receipt). Any gain or loss on a subsequent conversion or other disposition of such Canadian dollars by such U.S. Holder generally will be treated as ordinary income or loss and generally will be income or loss from sources within the United States for U.S. foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Distributions on the common shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute “passive category income.” Because we are not a United States corporation, such dividends will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations. Dividends paid by a “qualified foreign corporation” to a U.S. Holder who is an individual, trust or estate will generally be treated as “qualified dividend income” and are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are met. However, if we are a passive foreign investment company (PFIC) for the taxable year in which the dividend is paid or the preceding taxable year (see discussion below under “Passive Foreign Investment Company Considerations”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its own tax advisors regarding the availability of the reduced tax rate on dividends.
If a U.S. Holder is subject to Canadian withholding tax on dividends paid on the holder’s common shares (see discussion below under “Material Canadian Federal Income Tax Considerations—Dividends”), the U.S. Holder may be eligible, subject to a number of complex limitations, to claim a credit against its U.S. federal income tax for the Canadian withholding tax imposed on the dividends. However, if U.S. persons collectively own, directly or indirectly, 50% or more of the voting power or value of our common shares it is possible that a portion of any dividends we pay will be considered U.S. source income in proportion to our U.S. source earnings and profits, which could limit the ability of a U.S. Holder to claim a foreign tax credit for the Canadian withholding taxes imposed in respect of such a dividend, although certain elections may be available under the Code and the Convention to mitigate these effects. A U.S. Holder may claim a deduction for the Canadian withholding tax in lieu of a credit, but only for a year in which the U.S. Holder elects to do so for all creditable foreign income taxes. The rules governing the foreign tax credit are complex. Each U.S. Holder is advised to consult its tax advisor regarding the availability of the foreign tax credit under its particular circumstances.
Sale, Exchange or Other Disposition of Common Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of common shares. The amount of gain recognized will equal the excess of the amount realized (i.e., the amount of cash plus the fair market value of any property received) over the U.S. Holder’s adjusted tax basis in the common shares sold or exchanged. The amount of loss recognized will equal the excess of the U.S. Holder’s adjusted tax basis in the common shares sold or exchanged over the amount realized. Such capital gain or loss generally will be long-term capital gain or loss if, on the date of sale, exchange or other disposition, the common shares were held by the U.S. Holder for more than one year. Net long-term capital gain derived by a non-corporate U.S. Holder with respect to capital assets is currently is subject to tax at reduced rates. The deductibility of a capital loss is subject to limitations. Any gain or loss recognized from the sale, exchange or other disposition of common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes, except as otherwise provided in an applicable income tax treaty and if an election is properly made under the Code.
Passive Foreign Investment Company Considerations
In general, a corporation organized outside the United States will be treated as a PFIC in any taxable year in which either (1) at least 75% of its gross income is “passive income” or (2) at least 50% of the average quarterly value of its assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from commodities transactions and from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. The average percentage of a corporation’s assets that produce or are held for the production of passive income generally is determined on the basis of the fair market value of the corporation’s assets at the end of each quarter (which may be determined in part by the market value of our common shares, which is subject to change). In determining whether a foreign corporation is a PFIC, a proportionate share of the items of gross income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) are taken into account.
Although the tests for determining PFIC status are applied as of the end of each taxable year and are dependent upon a number of factors, some of which are beyond our control, including the value of our assets, the market price of our common shares, and the amount and type of our gross income (i) we believe that we were a PFIC for the taxable year ended December 31, 2016, and (ii) we do not believe that we were a PFIC for the taxable years ended December 31, 2019, 2018 and 2017. Our status as a PFIC is a fact-intensive determination made on an annual basis, and we cannot provide any assurance regarding our PFIC status for the taxable year ending December 31, 2020 or for subsequent taxable years. U.S. Holders who own our common shares for any period during which we are a PFIC will be required to file IRS Form 8621 for each tax year during which they hold our common shares. No opinion of legal counsel or ruling from the IRS concerning our status as a PFIC has been obtained or is currently planned to be requested. However, the determination of our PFIC status is made annually after the close of each taxable year and it is difficult to predict before such determination whether we will be a PFIC for any given taxable year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that the IRS will agree with our conclusion. No assurance can be provided regarding our PFIC status, and neither we nor our United States counsel expresses any opinion with respect to our PFIC status.
If we are a PFIC at any time when a non-corporate U.S. Holder owns common shares, and such U.S. Holder does not make a “qualified electing fund” election (QEF election) or a “mark-to-market” election, both as described below, such U.S. Holder will generally be subject to federal tax under the excess distribution rules (described below). Under such rules, additional taxes and interest charges would apply to certain distributions by us or to gain upon dispositions of our common shares. If neither of such elections are made, the excess distribution rules apply to (1) distributions paid during a taxable year that are greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the common shares, and (2) any gain recognized on a sale, exchange or other disposition (which would include a pledge or transfer by gift or death) of common shares. Under the excess distribution rules, the non-corporate U.S. Holder’s tax liability will be determined by allocating such distribution or gain ratably to each day in the U.S. Holder’s holding period for the common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we were a PFIC in the holding period will be taxed as ordinary income earned in the current taxable year and the preferential tax rate applicable to capital gains or dividends received on our common shares would not be available. The amount allocated to other taxable years (i.e., prior years in which we were a PFIC) will be taxed at the highest marginal rate in effect (for individuals or corporations as applicable) for ordinary income in each such taxable year, and an interest charge, generally applicable to the underpayment of tax, will be added to the tax and the preferential tax rate applicable to capital gains or dividends received on our common shares would not be available. These adverse tax consequences would not apply to a pension or profit-sharing trust or other tax-exempt organization that did not borrow funds or otherwise utilize leverage in connection with its acquisition of our common shares. In addition, if a non-electing U.S. Holder who is an individual dies while owning our common shares, such U.S. Holder's successor generally would not receive a step-up in tax basis with respect to such common shares, but instead would have a tax basis equal to the lower of the fair market value of such common shares or the decedent’s tax basis in such common shares.
If we are a PFIC at any time when a U.S. Holder holds our common shares, we will generally continue to be treated as a PFIC with respect to the U.S. Holder for all succeeding years during which the U.S. Holder holds our common shares even if we cease to meet the PFIC gross income test or asset test in a subsequent year. However, if we cease to meet these tests, a U.S. Holder can avoid the continuing impact of the PFIC rules by making a special election (a “Purging Election”) to recognize gain by making a “deemed sale” election with respect to all of the U.S. Holder’s common shares and have such common shares deemed to be sold at their fair market value on the last day of the last taxable year during which we were a PFIC. In addition, for a U.S. Holder making such an election, a new holding period would be deemed to begin for our common shares for purposes of the PFIC rules. After the Purging Election, the common shares with respect to which the Purging Election was made will not be treated as shares in a PFIC unless we subsequently again become a PFIC.
The tax considerations that would apply if we were a PFIC would be different from those described above if a U.S. Holder were able to make a valid QEF election. For each year that we meet the PFIC gross income test or asset test, an electing U.S. Holder would be required to include in gross income its pro rata share of our ordinary income and net capital gains, if any, as determined under U.S. federal income tax principles. The U.S. Holder’s adjusted tax basis in our common shares would be increased by the amount of such inclusions. An actual distribution to the U.S. Holder out of such income generally would not be treated as a dividend and would decrease the U.S. Holder’s adjusted tax basis in our common shares. Gain realized from the sale of our common shares covered by a QEF election would be taxed as a capital gain and the denial of the basis step-up at death described above would not apply. Generally, a QEF election must be made by the U.S. Holder in a timely filed tax return for the first taxable year in which the U.S. Holder held our common shares that includes the close of our taxable year for which we met the PFIC gross income test or asset test. A separate QEF election would need to be made for any of our subsidiaries that are classified as a PFIC. A QEF election is made on IRS Form 8621. U.S. Holders will be eligible to make QEF elections only if we agree to provide U.S. Holders with the information they will need to comply with the QEF rules. In the event we become a PFIC, we intend to provide all information and documentation that a U.S. Holder making a QEF election is required to obtain for U.S. federal income tax purposes (e.g., the U.S. Holder’s pro rata share of ordinary income and net capital gain, and a “PFIC Annual Information Statement” as described in applicable U.S. Treasury regulations).
As an alternative to a QEF election, a U.S. Holder may also mitigate the adverse tax consequences of PFIC status by timely making a “mark-to-market” election, provided the U.S. Holder completes and files IRS Form 8621 in accordance with the relevant instructions and related Treasury regulations. A U.S. Holder who makes the mark-to-market election generally must include as ordinary income each year the increase in the fair market value of the common shares and deduct from gross income the decrease in the value of such shares during each of its taxable years, but with losses limited to the amount of previously recognized net gains. The U.S. Holder’s tax basis in the common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. If a mark-to-market election with respect to our common shares is in effect on the date of a U.S. Holder’s death, the tax basis of the common shares in the hands of a U.S. Holder who acquired them from a decedent will be the lesser of the decedent’s tax basis or the fair market value of the common shares. Any gain from a sale, exchange or other disposition of the common shares in any taxable year in which we are a PFIC (i.e., when we meet the gross income test or asset test described above) would be treated as ordinary income and any loss from a sale, exchange or other disposition would be treated first as an ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as a capital loss. If we cease to be a PFIC, any gain or loss recognized by a U.S. Holder on the sale or exchange of the common shares would be classified as a capital gain or loss.
A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable U.S. Treasury regulations. A class of stock is regularly traded during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. The common shares should be marketable stock as long as they are listed on the Nasdaq Capital Market and are regularly traded. A mark-to-market election will not apply to the common shares for any taxable year during which we are not a PFIC but will remain in effect with respect to any subsequent taxable year in which we again become a PFIC. Such election will not apply to any subsidiary that we own. Accordingly, a U.S. Holder may continue to be subject to the PFIC rules with respect to any lower-tier PFICs notwithstanding the U.S. Holder’s mark-to-market election. Whether our common shares are regularly traded on a qualified exchange is an annual determination based on facts that, in part, are beyond our control. Accordingly, a U.S. Holder might not be eligible to make a mark-to-market election to mitigate the adverse tax consequences if we are characterized as a PFIC.
Each U.S. person who is a shareholder of a PFIC generally must file an annual report (on IRS Form 8621) with the IRS containing certain information, and the failure to file such report could result in the imposition of penalties on such U.S. person and in the extension of the statute of limitations with respect to federal income tax returns filed by such U.S. person.
The U.S. federal income tax rules relating to PFICs are very complex. U.S. Holders are urged to consult their own tax advisors with respect to the purchase, ownership and disposition of common shares, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of common shares in the event we are considered a PFIC.
Additional Tax on Passive Income
Certain U.S. Holders that are individuals, estates or trusts (other than trusts that are exempt from tax) with adjusted income exceeding certain thresholds, will be subject to a 3.8% tax on all or a portion of their “net investment income,” which includes dividends on the common shares, and net gains from the disposition of the common shares. Further, excess distributions treated as dividends, gains treated as excess distributions, and mark-to-market inclusions and deductions are all included in the calculation of net investment income.
Treasury regulations provide, subject to the election described in the following paragraph, that solely for purposes of this additional tax, that distributions of previously taxed income will be treated as dividends and included in net investment income subject to the additional 3.8% tax. Additionally, to determine the amount of any capital gain from the sale or other taxable disposition of common shares that will be subject to the additional tax on net investment income, a U.S. Holder who has made a QEF election will be required to recalculate its basis in the common shares excluding any QEF election basis adjustments.
Alternatively, a U.S. Holder may make an election which will be effective with respect to all interests in controlled foreign corporations and PFICs that are subject to a QEF election and that are held in that year or acquired in future years. Under this election, a U.S. Holder pays the additional 3.8% tax on QEF election income inclusions and on gains calculated after giving effect to related tax basis adjustments. U.S. Holders that are individuals, estates or trusts should consult their own tax advisors regarding the applicability of this tax to any of their income or gains in respect of the common shares.
U.S. Federal Income Taxation of Non-U.S. Holders
A beneficial owner of our common shares, other than a partnership or entity treated as a partnership for U.S. Federal income tax purposes, that is not a U.S. Holder is referred to herein as a “Non-U.S. Holder.” Non-U.S. Holders generally will not be subject to U.S. federal income tax or withholding tax on dividends received from us with respect to our common shares, unless that income is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States. In general, if the Non-U.S. Holder is entitled to the benefits of certain U.S. income tax treaties with respect to those dividends, that income is taxable only if it is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States.
Non-U.S. Holders generally will not be subject to U.S. federal income tax or withholding tax on any gain realized upon the sale, exchange or other disposition of our common shares, unless:
|
● |
the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States. In general, if the Non-U.S. Holder is entitled to the benefits of certain income tax treaties with respect to that gain, that gain is taxable only if it is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States; or |
|
● |
the Non-U.S. Holder is an individual who is present in the United States for 183 days or more during the taxable year of disposition and other conditions are met. |
If the Non-U.S. Holder is engaged in a U.S. trade or business for U.S. federal income tax purposes, the income from the common shares, including dividends and the gain from the sale, exchange or other disposition of the stock, that is effectively connected with the conduct of that trade or business will generally be subject to regular U.S. federal income tax in the same manner as discussed above relating to the general taxation of U.S. Holders. In addition, if you are a corporate Non-U.S. Holder, your earnings and profits that are attributable to the effectively connected income, which are subject to certain adjustments, may be subject to an additional branch profits tax at a rate of 30%, or at a lower rate as may be specified by an applicable U.S. income tax treaty.
Information Reporting with Respect to Foreign Financial Assets
U.S. individuals that own “specified foreign financial assets” (as defined in Section 6038D of the Code) with an aggregate fair market value exceeding certain threshold amounts generally are required to file an information report on IRS Form 8938 with respect to such assets with their tax returns. Significant penalties may apply to persons who fail to comply with these rules. Specified foreign financial assets include not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by certain financial institutions, any stock or security issued by a non-U.S. person, such as our common shares. Upon the issuance of future U.S. Treasury regulations, these information reporting requirements may apply to certain U.S. entities that own specified foreign financial assets. The failure to report information required under the current regulations could result in substantial penalties and in the extension of the statute of limitations with respect to federal income tax returns filed by a U.S. Holder. U.S. Holders should consult their own tax advisors regarding the possible implications of these U.S. Treasury regulations for an investment in our common shares.
Special Reporting Requirements for Transfers to Foreign Corporations
A U.S. Holder that acquires common shares generally will be required to file IRS Form 926 with the IRS if (1) immediately after the acquisition such U.S. Holder, directly or indirectly, owns at least 10% of our common shares, or (2) the amount of cash transferred in exchange for common shares during the 12-month period ending on the date of the acquisition exceeds US$100,000. Significant penalties may apply for failing to satisfy these filing requirements. U.S. Holders are urged to contact their tax advisors regarding these filing requirements.
Information Reporting and Backup Withholding
Dividends on and proceeds from the sale or other disposition of common shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if (1) the U.S. holder fails to provide an accurate taxpayer identification number or otherwise establish a basis for exemption, (2) the U.S. Holder is notified by the IRS that backup withholding applies, or (3) the payment is described in certain other categories of persons.
If you sell your common shares through a U.S. office of a broker, the payment of the proceeds is subject to both U.S. backup withholding and information reporting unless you certify that you are a non-U.S. person, under penalties of perjury, or you otherwise establish an exemption. If you sell your common shares through a non-U.S. office of a non-U.S. broker and the sales proceeds are paid to you outside the United States, then information reporting and backup withholding generally will not apply to that payment. However, U.S. information reporting requirements, but not backup withholding, will apply to a payment of sales proceeds, even if that payment is made to you outside the United States, if you sell your common shares through a non-U.S. office of a broker that is a U.S. person or has certain other contacts with the United States, unless you certify that you are a non-U.S. person, under penalty of perjury, or you otherwise establish an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder or Non-U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. U.S. Holders and Non-U.S. Holders should consult with their own tax advisors regarding their reporting obligations, if any, as a result of their acquisition, ownership, or disposition of our common shares.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A U.S. HOLDER. EACH U.S. HOLDER IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of August 5, 2020, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (Tax Act) generally applicable to a holder of our common shares who, for purposes of the Tax Act and at all relevant times, is neither resident in Canada nor deemed to be resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention, and who does not use or hold (and is not deemed to use or hold) common shares in the course of carrying on a business in Canada, deals at arm’s length with and is not affiliated with us and holds our common shares as capital property (Holder). Generally, common shares will be considered to be capital property to a Holder thereof provided that the Holder does not hold common shares in the course of carrying on a business and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder, (i) that is a “financial institution” for purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii) that holds an interest which is a “tax shelter investment” as defined in the Tax Act; or (iv) that has elected to report its tax results in a functional currency other than Canadian currency. Special rules, which are not discussed in this summary, may apply to a Holder that is an “authorized foreign bank” within the meaning of the Tax Act, a partnership or an insurer carrying on business in Canada and elsewhere. Such Holders should consult their own tax advisors.
This summary is based upon the provisions of the Tax Act (including the regulations (Regulations) thereunder) in force as of August 5, 2020 and our understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (CRA) published in writing by the CRA prior to August 5, 2020. This summary takes into account all specific proposals to amend the Tax Act (and the Regulations) publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (Tax Proposals) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action. This summary is not exhaustive of all possible Canadian federal income tax considerations and does not take into account other federal or any provincial, territorial or foreign income tax legislation or considerations, which may differ materially from those described in this summary.
This summary is of a general nature only and is not, and is not intended to be, and should not be construed to be, legal or tax advice to any particular Holder, and no representations concerning the tax consequences to any particular Holder are made. Holders should consult their own tax advisors regarding the income tax considerations applicable to them having regard to their particular circumstances.
Dividends
Dividends paid or credited (or deemed to be paid or credited) to a Holder by us are subject to Canadian withholding tax at the rate of 25% unless reduced by the terms of an applicable tax treaty or convention. For example, under the US Treaty, as amended, the dividend withholding tax rate is generally reduced to 15% in respect of a dividend paid or credited to a Holder beneficially entitled to the dividend who is resident in the United States for purposes of the US Treaty and whose entitlement to the benefits of the US Treaty is not limited by the limitation of benefits provisions of the US Treaty. Holders are urged to consult their own tax advisors to determine their entitlement to relief under the US Treaty or any other applicable tax treaty as well as their ability to claim foreign tax credits with respect to any Canadian withholding tax, based on their particular circumstances.
Disposition of Common Shares
A Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a common share, unless the common share constitutes or is deemed to constitute “taxable Canadian property” to the Holder thereof for purposes of the Tax Act, and the gain is not exempt from tax pursuant to the terms of an applicable tax treaty or convention.
In general, provided the common shares are listed on a “designated stock exchange” (which currently includes the Nasdaq Capital Market) at the date of the disposition, the common shares will only constitute “taxable Canadian property” of a Holder if, at any time within the 60-month period preceding the disposition: (i) such Holder, persons with whom the Holder did not deal at arm’s length, partnerships in which the Holder or a person with whom the Holder did not deal at arm’s length holds a membership interest directly or indirectly through one or more partnerships, or any combination thereof, owned 25% or more of the issued shares of any class or series of the Company’s share capital; and (ii) more than 50% of the fair market value of the common shares was derived directly or indirectly from one or any combination of (A) real or immovable property situated in Canada, (B) Canadian resource properties, (C) timber resource properties, and (D) options in respect of, or interests in, or for civil law rights in, property described in any of subparagraphs (ii)(A) to (C), whether or not the property exists. However, and despite the foregoing, in certain circumstances the common shares may be deemed to be “taxable Canadian property” under the Tax Act.
Holders whose common shares may be “taxable Canadian property” should consult their own tax advisers.
UNDERWRITING
Guggenheim Securities, LLC is acting as representative of each of the underwriters named below. Subject to the terms and conditions set forth in the underwriting agreement between us and the underwriters, each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of common shares set forth opposite its name below.
|
Underwriter |
|
Number of Shares |
|
Guggenheim Securities, LLC |
|
|
| Craig-Hallum Capital Group | ||
|
Total |
|
|
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares subject to their acceptance of the comon shares from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts; Expenses
The underwriters have advised us that they propose initially to offer the shares to the public at the public offering price set forth on the cover of this prospectus supplement and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional common shares from us, as applicable.
|
Total |
|||||
|
Per Share |
No Exercise |
Full Exercise |
|||
|
Public offering price |
|||||
|
Underwriting discounts and commissions to be paid by us |
|||||
|
Proceeds, before expenses, to us |
|||||
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $ , which includes certain expenses incurred by the underwriters in connection with this offering that will be reimbursed by us. We have agreed to reimburse the underwriters for certain expenses incurred by them in connection with this offering (including certain fees and expenses of counsel for the underwriters and fees and expenses related to filings with and review by FINRA) in an amount not to exceed $150,000.
Option to Purchase Additional Shares
We have granted the underwriters an option to purchase up to an additional common shares at the public offering price, less the underwriting discounts and commissions, within 30 days from the date of this prospectus supplement. If the underwriters exercise this option, each underwriter will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
No Sales of Similar Securities
In connection with this offering, we have agreed with the underwriters that, subject to certain customary exceptions, without the prior written consent of Guggenheim Securities, LLC on behalf of the underwriters, we will not, for a period ending 90 days after the date of this prospectus supplement (the Lock-Up Period) (a) directly or indirectly, issue, offer, sell, agree to issue, offer or sell, solicit offers to purchase, grant any call option, warrant or other right to purchase, purchase any put option or other right to sell, pledge, hypothecate, borrow or otherwise transfer or dispose of any common shares or any securities convertible into or exercisable or exchangeable for common shares, or make any public announcement of any of the foregoing, (b) establish or increase any “put equivalent position” or liquidate or decrease any “call equivalent position” (in each case within the meaning of Section 16 of the Exchange Act and the rules and regulations thereunder) with respect to any common shares or any securities convertible into or exercisable or exchangeable for common shares, (c) otherwise enter into any swap, derivative or other transaction or arrangement that transfers to another, in whole or in part, any economic consequence of ownership of any common shares or any securities convertible into or exercisable or exchangeable for common shares, whether or not such transaction is to be settled by delivery of any common shares, securities convertible into or exercisable or exchangeable for common shares, other securities, cash or other consideration, (d) submit or file any registration statement under the Securities Act in respect of any common shares or any securities convertible into or exercisable or exchangeable for our common shares, or (e) publicly announce the intention to do any of the foregoing.
In connection with this offering, certain of our stockholders and our directors and executive officers have agreed with the underwriters that, subject to certain customary exceptions, without the prior written consent of Guggenheim Securities, LLC on behalf of the underwriters, they will not, for the Lock-Up Period, (a) directly or indirectly, offer, sell, agree to offer or sell, solicit offers to purchase, grant any call option or purchase any put option with respect to, pledge, borrow or otherwise dispose of, any common shares and any security convertible into, or exercisable or exchangeable for, common shares (Relevant Security), (b) establish or increase any “put equivalent position” or liquidate or decrease any “call equivalent position” with respect to any Relevant Security (in each case within the meaning of Section 16 of the Exchange Act, and the rules and regulations promulgated thereunder), or otherwise enter into any swap, derivative or other transaction or arrangement that transfers to another, in whole or in part, any economic consequence of ownership of a Relevant Security, whether or not such transaction is to be settled by delivery of Relevant Securities, other securities, cash or other consideration, (c) make any demand for, or exercise any right with respect to, the registration under the Securities Act of the offer and sale of any Relevant Security, or cause to be filed a registration statement, prospectus or prospectus supplement (or an amendment or supplement thereto) with respect to any such registration or (d) publicly announce any intention to do any of the foregoing. The underwriters may, in their sole discretion, permit the sale of Relevant Securities during the restricted period in whole or in part and at any time, with or without notice.
Nasdaq Capital Market Listing
Our common shares are listed and trade in the United States on the Nasdaq Capital Market under the trading symbol “DMAC.”
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common shares. However, the representative may engage in transactions that stabilize the price of the common shares, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common shares in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option described above. The underwriters may close out any covered short position by either exercising their option or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option granted to them. “Naked” short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common shares in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common shares made by the underwriters in the open market prior to the closing of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common share or preventing or retarding a decline in the market price of our common shares. As a result, the price of our common shares may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the Nasdaq Capital Market, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common shares. In addition, neither we nor any of the underwriters make any representation that the representative will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Passive Market Making
Any underwriters who are qualified market makers on the Nasdaq Capital Market may engage in passive market making transactions in the securities on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Right of First Refusal
In connection with this offering, we have granted to Guggenheim Securities, LLC a right of first refusal for a period of 12 months from the date of closing of this offering to act as left lead bookrunner in connection with any public offering of our equity securities, other than an at-the-market offering.
Other Relationships
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Some of the underwriters and certain of their affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us and our affiliates, for which they may in the future receive customary fees, commissions and expenses.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers.
Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area, or a Member State, no shares have been offered or will be offered pursuant to the offering to the public in that Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent authority in that Member State, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
|
A |
to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
|
B. |
to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the underwriters; or |
|
C. |
in any other circumstances falling within Article 1(4) of the Prospectus Regulation; |
provided that no such offer of shares shall require the Company or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the Company that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation.
In the case of any shares being offered to a financial intermediary as that term is used in Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Member State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer to the public” in relation to shares in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
MiFID II Product Governance
Any person offering, selling or recommending the shares, or a distributor, should take into consideration the manufacturers’ target market assessment; however, a distributor subject to MiFID II is responsible for undertaking its own target market assessment in respect of the shares (by either adopting or refining the manufacturers’ target market assessment) and determining appropriate distribution channels.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Regulation) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the Order) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Canada
The securities subject to this offering are not qualified for sale in Canada and may not be offered or sold in Canada, directly or indirectly, on our behalf. Purchasers of the securities subject to this offering are hereby notified that their purchase of securities subject to this offering will be deemed to constitute a representation and warranty that such investor is not a Canadian resident and is purchasing the shares with investment intent and not for the purposes of making an immediate resale in Canada.
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
LEGAL MATTERS
The validity of the common shares offered hereby by us will be passed upon for us by Pushor Mitchell LLP, Kelowna, British Columbia, Canada, relating to matters of British Columbia law, and Fox Rothschild LLP, New York, New York, relating to matters of New York law. Certain legal matters in connection with this offering will be passed upon for the underwriters by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts.
EXPERTS
The consolidated financial statements incorporated into this prospectus supplement by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 have been audited by Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm. Their report, which is incorporated herein by reference, expresses an unqualified opinion on the consolidated financial statements. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference the information we file with them. This allows us to disclose important information to you by referencing those filed documents. We have previously filed the documents set forth below with the SEC and are incorporating them by reference into this prospectus supplement. Our SEC file no. is 001-36291.
|
● |
Annual Report on Form 10-K for the year ended December 31, 2019; |
|
● |
Definitive Proxy Statement for our 2020 Annual General Meeting of Shareholders as filed with the SEC on April 20, 2020 (but only with respect to information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2019); |
|
● |
Quarterly Report on Form 10-Q for the quarter ended March 31, 2020; |
|
● |
Current Reports on Form 8-K (only to the extent information is “filed” and not “furnished”) filed with the SEC on January 3, 2020, February 11, 2020, February 13, 2020, May 13, 2020, and June 3, 2020; and |
|
● |
the description of our common shares contained in our Amendment No. 1 to our registration statement on Form 8-A that we filed with the SEC on June 4, 2019, and any amendment or report filed for the purpose of updating this description. |
We also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus supplement is a part and before the effective date of the registration statement and after the date of this prospectus supplement until the termination of the offering. The most recent information that we file with the SEC automatically updates and supersedes more dated information.
You may access our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statement, and amendments, if any, to those documents filed or furnished pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act with the SEC free of charge at the SEC’s website at www.sec.gov or our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
You can obtain a copy of any documents, which are incorporated by reference in this prospectus supplement or prospectus supplement, except for exhibits which are not specifically incorporated by reference into those documents, at no cost, by writing or telephoning us at:
DiaMedica Therapeutics Inc.
Two Carlson Parkway, Suite 260
Minneapolis, Minnesota 55447
Attention: Secretary
(763) 312-6755
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered by this prospectus supplement. When used in this prospectus supplement, the term “registration statement” includes amendments to the registration statement as well as the exhibits, schedules, financial statements and notes filed as part of the registration statement. This prospectus supplement and the accompanying prospectus, which constitutes a part of the registration statement, does not contain all of the information in the registration statement. This prospectus supplement and accompanying prospectus omits information contained in the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and the common shares and other securities that may be offered by this prospectus supplement, reference is made to the registration statement. Statements herein concerning the contents of any contract or other document are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed with the SEC as an exhibit to the registration statement, each such statement being qualified by and subject to such reference in all respects.
In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the Internet at the SEC’s website at www.sec.gov.
We also file annual audited and interim unaudited financial statements, proxy statements and other information with the Ontario, Manitoba, Québec, Alberta and British Columbia Securities Commissions. Copies of these documents that are filed through the System for Electronic Document Analysis and Retrieval of the Canadian Securities Administrators are available at its website http://www.sedar.com.
In addition, we maintain a website that contains information regarding our company, including copies of reports, proxy statements and other information we file with the SEC. The address of our website is www.diamedica.com. Except for the documents specifically incorporated by reference into this prospectus supplement, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus supplement. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
PROSPECTUS

$50,000,000
Common Shares
Warrants
Units
We may from time to time offer to sell any combination of common shares, warrants and units described in this prospectus in one or more offerings. The aggregate initial offering price of all securities sold under this prospectus will not exceed $50,000,000.
This prospectus provides a general description of the securities that we may offer. Each time we sell securities, we will provide the specific terms of the securities offered in a supplement to this prospectus. The prospectus supplement may also add, update or change information contained in this prospectus. You should read this prospectus and the applicable prospectus supplement carefully before you invest in any securities. This prospectus may not be used to consummate a sale of securities unless accompanied by the applicable prospectus supplement.
We may from time to time offer and sell our securities in one offering or in separate offerings, to or through underwriters, dealers and agents or directly to purchasers. If any agents or underwriters are involved in the sale of any of these securities, the applicable prospectus supplement will provide the names of the agents or underwriters and any applicable fees, commissions or discounts.
Our common shares are listed on The Nasdaq Capital Market under the symbol “DMAC.” On December 31, 2019, the closing price of our common shares as reported on The Nasdaq Capital Market was $4.85 per share. As of the date of this prospectus, the aggregate market value of our common shares held by non-affiliates pursuant to General Instruction I.B.6 of Form S-3 is $52,130,710, which is calculated based on 10,748,600 common shares outstanding held by non-affiliates and a price of $4.85 per share, the closing price of our common shares on December 31, 2019, as reported on The Nasdaq Capital Market. During the prior 12 calendar month period that ends on and includes the date hereof, we have not offered or sold any of our common shares or other securities pursuant to General Instruction I.B.6 to Form S-3. Pursuant to General Instruction I.B.6 to Form S-3, in no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million.
We are an “emerging growth company,” as defined under federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements. See “About the Company – Implications of Being an Emerging Growth Company and Smaller Reporting Company” beginning on page 3 of this prospectus.
Investing in our securities involves risks. You should consider carefully the risks and uncertainties set forth in the section entitled “Risk Factors” beginning on page 4 of this prospectus, in the related prospectus supplement, and in the documents we file with the Securities and Exchange Commission that are incorporated by reference in this prospectus before making a decision to purchase our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is January 9, 2020
TABLE OF CONTENTS
Page
|
ABOUT THIS PROSPECTUS |
1 |
|
ABOUT THE COMPANY |
2 |
|
RISK FACTORS |
4 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
4 |
|
USE OF PROCEEDS |
5 |
|
DILUTION |
5 |
|
DESCRIPTION OF OUR COMMON SHARES |
6 |
|
DESCRIPTION OF WARRANTS |
11 |
|
DESCRIPTION OF UNITS |
12 |
|
PLAN OF DISTRIBUTION |
13 |
|
LEGAL MATTERS |
14 |
|
EXPERTS |
14 |
|
WHERE YOU CAN FIND MORE INFORMATION |
15 |
|
INCORPORATION OF DOCUMENTS BY REFERENCE |
15 |
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the United States Securities and Exchange Commission (SEC) utilizing a “shelf” registration process. Under this shelf registration process, we may offer and sell any combination of the securities described in this prospectus in one or more offerings up to a total dollar amount of $50,000,000.
This prospectus provides you with a general description of the respective securities that we may offer. Each time we sell securities under this shelf registration statement, we will provide a prospectus supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus. To the extent that any statement that we make in a prospectus supplement is inconsistent with statements made in this prospectus, the statements made in this prospectus will be deemed modified or superseded by those made in the prospectus supplement. You should read both this prospectus and the accompanying prospectus supplement, including all documents incorporated herein or therein by reference, together with additional information described under “Where You Can Find More Information” and “Incorporation of Documents by Reference.”
We have not authorized any dealer, salesperson or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus and the accompanying prospectus supplement. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or the accompanying prospectus supplement. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and the accompanying prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus and the accompanying prospectus supplement is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.
This prospectus may not be used to offer and sell securities unless it is accompanied by an additional prospectus or a prospectus supplement.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “DiaMedica,” “the Company,” “we,” “us,” “our” or similar references mean DiaMedica Therapeutics Inc. and its subsidiaries. References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value per share.
All references in this prospectus to “$,” “U.S. Dollars” and “dollars” are to United States dollars.
We own various unregistered trademarks and service marks, including our corporate logo. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the owner of such trademarks and trade names will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
ABOUT THE COMPANY
Overview
We are a clinical stage biopharmaceutical company primarily focused on the development of novel recombinant proteins. Our goal is to use our trade secrets and patented and licensed technologies to establish our company as a leader in the development and commercialization of therapeutic treatments derived from novel recombinant proteins. Our current focus is on chronic kidney disease (CKD) and acute ischemic stroke (AIS). We plan to advance DM199, our lead drug candidate, through required clinical trials to create shareholder value by establishing its clinical and commercial potential as a therapy for CKD and AIS.
DM199 is a recombinant form of human tissue kallikrein-1 (KLK1). KLK1 is a serine protease (protein) produced primarily in the kidneys, pancreas and salivary glands that plays a critical role in the regulation of local blood flow and vasodilation (the widening of blood vessels, which decreases blood pressure) in the body, as well as an important role in inflammation and oxidative stress (an imbalance between potentially damaging reactive oxygen species, or free radicals, and antioxidants in your body). We believe DM199 has the potential to treat a variety of diseases where healthy functioning requires sufficient activity of KLK1 and its system, the kallikrein-kinin system (KKS).
AIS and CKD patients suffer from a lack of blood flow to the brain and kidneys, respectively. These patients also tend to exhibit lower than normal levels of endogenous KLK1. We believe treatment with DM199 could replenish low levels of endogenous KLK1, thereby releasing physiological levels of bradykinin (BK) when and where needed, generating beneficial nitric oxide and prostacyclin, setting in motion metabolic pathways that can improve blood flow (through vasoregulation) to damaged end-organs, such as the brain and kidneys, supporting structural integrity and normal functioning.
Today, forms of KLK1 derived from human urine and porcine pancreas are sold in Japan, China and Korea to treat AIS, CKD, retinopathy, hypertension and related vascular diseases. We believe millions of patients have been treated with these KLK1 therapies, and the data from more than 100 published papers and studies support their clinical benefit. However, there are numerous regulatory, commercial, and clinical drawbacks associated with KLK1 derived from human urine and porcine pancreas that can be overcome by developing a synthetic version of KLK1 such as DM199. We believe regulatory drawbacks are the primary reason why KLK1 derived from human urine and porcine pancreas are not currently available and used in the United States or Europe. We are not aware of any synthetic version of KLK1 with regulatory approval for human use in any country, nor are we aware of any synthetic version in development other than our drug candidate DM199. We believe at least five companies have attempted to create a synthetic version of KLK1, but have been unsuccessful.
In July 2019, we completed a Phase Ib clinical trial of DM199 in participants with moderate or severe CKD caused by Type I or Type II diabetes. We initiated dosing patients in this study in February 2019 and completed enrollment in July 2019. The study was performed to assess the pharmacokinetics (PK) of three dose levels of DM199 (3, 5 and 8 µg/kg), administered in a single subcutaneous dose, as well as the evaluation of safety, tolerability and secondary pharmacodynamic (PD) endpoints. The study results demonstrated that at the 3µg/kg dose level, the PK profiles were similar between moderate and severe CKD patients, and consistent with healthy subjects (normal kidney function) tested previously, and that DM199 was well tolerated with no dose-limiting tolerability. There were no deaths, no discontinuations due to a treatment-related adverse event (AE), and no treatment-related significant adverse events (SAEs). AEs were minor and consistent with standard treatment(s) in the CKD patient population. In addition, favorable overall PD results were also observed, including short-term improvements in Nitric Oxide (NO), average increase of 35.2%, Prostaglandin E2 (PGE2), average increase of 41.2%, estimated glomerular flow rate (eGFR), average increase of 4.08 mL/min/1732, and the urinary albumin to creatinine ratio (UACR), average decrease of 18.7%. PD results appeared to be drug related in that the greatest improvements occurred approximately 24 hours after DM199 administration and subsequently declined.
In December 2019, we began enrolling patients in a Phase II CKD trial named REDUX, Latin for restore, a multi-center, open-label investigation of approximately 60 participants with CKD, who are being enrolled in two cohorts (30 per cohort). The study is being conducted in the United States at up to 10 sites and will be focused on participants with CKD. Cohort I of the study is focused on non-diabetic, hypertensive African Americans with Stage II or III CKD. African Americans are at greater risk for CKD than Caucasians, and those who have the APOL1 gene mutation are at an even higher risk. The study is designed to capture the APOL1 gene mutation as an exploratory biomarker in this cohort. Cohort II of the study is focused on participants with IgA Nephropathy (IgAN). The study will evaluate two dose levels of DM199 within each cohort. Study participants will receive DM199 by subcutaneous injection twice weekly for 95 days. The primary study endpoints include safety, tolerability, blood pressure, proteinuria and kidney function, which will be evaluated by changes from baseline in eGFR and albuminuria, as measured by the UACR.
In October 2019, we completed enrollment in the REMEDY trial, the Company’s Phase II study assessing the safety, tolerability and markers of therapeutic efficacy of DM199 in participants suffering from AIS. Final enrollment was 92 participants. The markers of therapeutic efficacy will include multiple plasma-based biomarkers (e.g. C-reactive protein), the Modified Rankin Scale, National Institute of Health Stroke Scale and the Barthel Index. These markers are assessed at multiple points throughout the study, including 90 days post-stroke.
Implications of Being an Emerging Growth Company and Smaller Reporting Company
As a company with less than $1.07 billion of revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act), and we may remain an emerging growth company for up to five years from December 31, 2018. However, if certain events occur prior to the end of such five-year period, including if we become a large accelerated filer, our annual gross revenue exceeds $1.07 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. In particular, we are required to provide only two years of audited financial statements and are not required to disclose all of the executive compensation related information that would be required if we were not an emerging growth company. Accordingly, the information contained in our SEC reports may be different than the information you receive from other public companies in which you hold equity interests. However, we have irrevocably elected not to avail ourselves of the extended transition period for complying with new or revised accounting standards, and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We are also a “smaller reporting company” as defined in the United States Securities Exchange Act of 1934, as amended (Exchange Act). We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies until the fiscal year following the determination that our voting and non-voting common shares held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are more than $100 million during the most recently completed fiscal year and our voting and non-voting common shares held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter.
Corporate Information
Our principal executive offices are located at 2 Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447. Our telephone number is (763) 312-6755, and our Internet website address is www.diamedica.com. We make available on our website free of charge a link to our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as practicable after we electronically file such material with the SEC. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We are a corporation governed under the British Columbia Business Corporations Act. Our company was initially incorporated under the name Diabex Inc. pursuant to The Corporations Act (Manitoba) by articles of incorporation dated January 21, 2000. Our articles were amended (i) on February 26, 2001 to change our corporate name to DiaMedica Inc., (ii) on April 11, 2016 to continue the Company from The Corporations Act (Manitoba) to the CBCA, (iii) on December 28, 2016 to change our corporate name to DiaMedica Therapeutics Inc., (iv) on September 24, 2018 to permit us to hold shareholder meetings in the U.S. and to permit our directors, between annual general meetings of our shareholders, to appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of shareholders, (v) on November 15, 2018 to effect a 1-for-20 consolidation of our common shares, and (vi) on May 31, 2019, to continue our existence from a corporation incorporated under the Canada Business Corporations Act into British Columbia under British Columbia’s Business Corporations Act.
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described in our filings with the SEC referred to under the heading “Where You Can Find More Information,” including the risk factors incorporated by reference herein from our most recent annual report on Form 10-K and quarterly reports on Form 10-Q and from other reports and documents we file with the SEC after the date of this prospectus that are incorporated by reference herein, together with all of the other information included in this prospectus, the applicable prospectus supplement and the documents we incorporate by reference.
If any of these risks were to occur, our business, financial condition, results of operations or cash flows could be adversely affected. You could lose all or part of your investment. When we offer and sell any securities pursuant to a prospectus supplement, we may include additional risk factors relevant to that offering in the prospectus supplement.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this prospectus and the related prospectus supplement that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology, and the use of future dates.
The forward-looking statements in or incorporated by reference into this prospectus or the related prospectus supplement may include, among other things, statements about:
|
● |
our plans to develop, obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of CKD and AIS and our expectations regarding the benefits of our DM199 product candidate; |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain required regulatory approvals of our DM199 product candidate for CKD and AIS; |
|
● |
the perceived benefits of our DM199 product candidate over existing treatment options for CKD and AIS; |
|
● |
the potential size of the markets for our DM199 product candidate and our ability to serve those markets; |
|
● |
the rate and degree of market acceptance, both in the United States and internationally, of our DM199 product candidate for CKD and AIS; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for and commercialize our DM199 product candidate for CKD and AIS, and any adverse ramifications as a result of our termination of a license and collaboration agreement with Ahon Pharmaceutical Co., Ltd.; |
|
● |
the success, cost and timing of planned clinical trials, as well as our reliance on collaboration with third parties to conduct our clinical trials; |
|
● |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
● |
expectations regarding federal, state, and foreign regulatory requirements and developments, such as potential United States Food and Drug Administration (FDA) regulation of our DM199 product candidate for CKD and AIS; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for CKD and AIS; |
|
● |
our ability to obtain funding for our operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for our DM199 product candidate for CKD and AIS; |
|
● |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; and |
|
● |
our anticipated use of the net proceeds from our December 2018 initial public offering in the United States and any offering under this prospectus and the related prospectus supplement to be filed in connection with such offering. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under “Risk Factors” in this prospectus and related prospectus supplement. Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Except as required by law, including the securities laws of the United States, we do not intend to update any forward-looking statements to conform these statements to actual results or to changes in our expectations.
USE OF PROCEEDS
Unless otherwise indicated in the prospectus supplement, we intend to use the net proceeds from the sale of securities and any exercise of warrants under this prospectus and related prospectus supplement to continue our clinical and product development activities and for other working capital and general corporate purposes. The prospectus supplement relating to a particular offering of securities by us will identify the use of proceeds for that offering. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the proceeds. Pending the uses described above, we intend to deposit the proceeds in our non-interest bearing checking account, U.S. Treasury money market fund or invest them temporarily in short-term or marketable securities until we use them for their stated purpose.
DILUTION
We will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors purchasing securities in an offering under this prospectus:
|
● |
the net tangible book value per share of our equity securities before and after the offering; |
|
● |
the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and |
|
● |
the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers. |
DESCRIPTION OF OUR COMMON SHARES
General
The following is a summary of the material terms of our common shares, as well as other material terms of our Notice of Articles and Articles and certain provisions of the British Columbia Business Corporations Act (BCBCA). References in this prospectus to “voting common shares” or “common shares” mean our voting common shares, no par value. This summary does not purport to be complete and is qualified in its entirety by the provisions of our Notice of Articles and Articles, which are included as exhibits to the registration statement of which this prospectus forms a part. For more information on how you can obtain our Notice of Articles and Articles, see the heading “Where You Can Find Additional Information.”
Authorized Share Capital
We have an authorized share capital consisting of an unlimited number of common shares, no par value per share.
Outstanding Common Shares
As of December 31, 2019, there were 12,006,874 common shares issued and outstanding. As of December 31, 2019, the following additional common shares were reserved for issuance:
|
● |
766,953 common shares were reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.65 per share; |
|
● |
605,181 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. Stock Option Plan, with a weighted average exercise price of $6.09 per share; |
|
● |
21,183 common shares were reserved for issuance upon the settlement of deferred share units outstanding under the DiaMedica Therapeutics Inc. Deferred Share Unit Plan; |
|
● |
615,178 common shares were reserved for issuance upon exercise of outstanding stock options under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan, with a weighted average exercise price of $4.55 per share; and |
|
● |
1,384,822 common shares were reserved for future issuance in connection with future grants under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. |
Certain Rights of the Common Shares
Dividends
Holders of our common shares are entitled to share pro rata in such dividends as may be declared by our Board of Directors. Pursuant to the provisions of the BCBCA, we may not declare or pay a dividend if there are reasonable grounds for believing that we are, or would after the payment be, unable to pay our liabilities as they become due in the ordinary course of business. We may pay a dividend by issuing fully paid shares, bonds, debentures or other of our securities or in property (including money).
Liquidation, Dissolution or Winding-Up
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company or any other distribution of our assets among our shareholders for the purpose of winding-up our affairs, holders of common shares are entitled to share pro rata in our assets available for distribution after we pay our creditors.
Voting Rights and Shareholders’ Meetings
Holders of our common shares are entitled to receive notice of and to attend and vote at all meetings of our shareholders. Each holder of our common shares is entitled to one vote, either in person or by proxy, on all matters submitted to shareholders.
Our Board of Directors must call an annual general meeting of shareholders to be held not later than 15 months after the last preceding annual general meeting of shareholders but no later than six months after the end of our preceding financial year end and may, at any time, call a special meeting of shareholders. Under our articles, a meeting of our shareholders may be held anywhere in or outside of British Columbia, as determined by the Board of Directors. For purposes of determining the shareholders who are entitled to receive notice of or to vote at a meeting of shareholders, the Board of Directors may, in accordance with National Instrument 54-101 - Communications with Beneficial Owners of Securities of a Reporting Issuer of the Canadian Securities Administrators, fix in advance a date as the record date for that determination of shareholders, but that record date may not be more than 60 days or less than 30 days before the date on which the meeting is to be held.
Our Articles provide that notice of the time and place of a meeting of shareholders must be sent to each shareholder entitled to vote at the meeting, each director and to our auditors, not more than 50 days and not less than 21 days prior to the meeting. Under our Articles, the presence at a shareholder meeting, in person or represented by proxy, of any number of shareholders holding not less than 33 1/3 of the issued common shares shall constitute a quorum for the purpose of transacting business at the shareholder meeting. A shareholder may participate in a meeting by means of telephone or other communication medium that permits all persons participating in the meeting to communicate with each other during the meeting.
In the case of joint shareholders, one of the holders present at a meeting, either personally or by proxy, may, in the absence of the other holder(s) of the shares, vote the shares. If two or more joint shareholders are present, personally or by proxy, then only the vote of the joint shareholder present whose name stands first on the central securities register in respect of the share will be counted.
No Preemption Rights; Limited Restrictions on Directors’ Authority to Issue Common Shares
Existing holders of our common shares have no rights of preemption or first refusal under our Articles or the BCBCA with respect to future issuances of our common shares. The common shares do not have conversion rights, are not subject to redemption and do not have the benefit of any sinking fund provisions. Subject to the rules and policies of The Nasdaq Stock Market and applicable corporate and securities laws, our Board of Directors has the authority to issue additional common shares.
Amendments to Articles
The Articles and the BCBCA govern the rights of holders of our common shares.
Subject to the BCBCA, unless an alteration to the Company’s Notice of Articles would be required, our directors can authorize the alteration of our Articles to, among other things, create additional classes or series of shares or, if none of the shares of a class or series are allotted or issued, eliminate that class or series of shares.
Subject to the BCBCA, our shareholders can authorize the alteration of our Articles and Notice of Articles to create or vary the rights or restrictions attached to any class of our shares by passing an ordinary resolution at a duly convened meeting of shareholders. An alteration to the Company’s Notice of Articles will not be effective until the notice of alteration is filed with the registrar pursuant to the BCBCA. An alteration to the Company’s Articles, which is not an alteration to the Company’s Notice of Articles, will be effective on the date and time that the resolution is received for deposit at the Company’s records office.
Fundamental Changes
Pursuant to the BCBCA, we may not effect any of the following fundamental changes without the consent of the holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and separately as a class at a duly convened meeting of our shareholders:
|
● |
any proposed amalgamation involving our company in respect of which the BCBCA requires that the approval of our shareholders be obtained; |
|
● |
any proposed plan of arrangement pursuant to the BCBCA involving our company in respect of which the BCBCA or any order issued by an applicable court requires that the approval of our shareholders be obtained; |
|
● |
any proposed sale, lease or exchange of all or substantially all of our undertaking; and |
|
● |
any voluntary liquidation of our company. |
Election and Removal of Directors
At each annual general meeting of shareholders, our shareholders are required to elect directors to hold office for a term expiring not later than the close of the next annual general meeting of shareholders. Our Board of Directors may fill vacancies among the Board. Our directors may also, between annual general meetings of our shareholders, appoint one or more additional directors to serve until the next annual general meeting of shareholders; provided, however, that the number of additional directors shall not at any time exceed one-third (1/3) of the number of directors who held office at the expiration of the last meeting of shareholders.
Since shareholders do not have cumulative voting rights, holders of more than 50% of our outstanding common shares can elect all of our directors if they choose to do so. In such event, holders of the remaining shares will be unable to elect any director.
Under the BCBCA, a public company must have a minimum of three directors, who are not required to be resident Canadians.
Under the BCBCA, a director may be removed by shareholders by special resolution unless the Articles provide for a lower approval level. The Articles allow shareholders to remove directors by a special resolution if approved by holders of at least two-thirds (2/3) of each class of our outstanding common shares represented in person or by proxy and voting separately as a class at a duly convened meeting of our shareholders.
Registration Rights
We have not granted any rights to have our common shares or other securities registered under the United States Securities Act of 1933, as amended (Securities Act).
Listing
Our common shares are listed and trade in the United States on The Nasdaq Capital Market under the trading symbol “DMAC.”
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is Computershare Investor Services.
Limitation of Liability and Indemnification Matters
Our Articles provide that we will indemnify our directors, former directors, his or her heirs and legal personal representatives and other individuals as we may determine against all eligible penalties to which such person is or may be liable to the fullest extent permitted by British Columbia law. We will pay all expenses actually and reasonably incurred by such person, either as such expenses are incurred in advance of the final disposition of an eligible proceeding or after the final disposition of an eligible proceeding. British Columbia law provides that a company must not indemnify its directors if any of the following circumstances apply:
|
● |
if the indemnity or payment is made under an earlier agreement to indemnify or pay expenses and, at the time that the agreement to indemnify or pay expenses was made, the company was prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if the indemnity or payment is made otherwise than under an earlier agreement to indemnify or pay expenses and, at the time that the indemnity or payment is made, the company is prohibited from giving the indemnity or paying the expenses by its articles; |
|
● |
if, in relation to the subject matter of the relevant proceeding, the director did not act honestly and in good faith with a view to the best interests of the company or the associated corporation, as the case may be, with such associated corporation being an affiliate of the company or a partnership, trust, joint venture or other unincorporated entity in which the director served in the capacity as a director or a position equivalent to that thereof, at the request of the company; or |
|
● |
in the case of the relevant proceeding other than a civil proceeding, if the director did not have reasonable grounds for believing that the director’s conduct in respect of which the proceeding was brought was lawful. |
Notwithstanding any of the above prohibitions, the company or a director may apply to court for an order that the company must indemnify the director for any liability or expenses incurred by the director or for any other related obligations of the company.
The Articles also permit us to purchase insurance on behalf of any officer, director, employee or other agent of our company, of an affiliated entity, or, at our request, of another entity, for any liability arising out of that person’s actions in such capacity. We have entered into indemnification agreements with each of our current directors and executive officers requiring us to indemnify these individuals to the fullest extent permitted under British Columbia law against liability that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified, and have received a written undertaking from each such director and officer as required under British Columbia law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Shareholder Rights Plan
We adopted a shareholder rights plan agreement (Rights Plan). The Rights Plan is designed to provide adequate time for the Board of Directors and the shareholders to assess an unsolicited takeover bid for DiaMedica, to provide the Board of Directors with sufficient time to explore and develop alternatives for maximizing shareholder value if a takeover bid is made, and to provide shareholders with an equal opportunity to participate in a takeover bid and receive full and fair value for their common shares. The Rights Plan was renewed at the Company’s annual general meeting of shareholders in December 2017 and is set to expire at the close of the Company’s annual general meeting of shareholders in 2020.
The rights issued under the Rights Plan will initially attach to and trade with the common shares, and no separate certificates will be issued unless an event triggering these rights occurs. The rights will become exercisable only when a person, including any party related to it, acquires or attempts to acquire 20% or more of the outstanding common shares without complying with the “Permitted Bid” provisions of the Rights Plan or without approval of the Board of Directors. Should such an acquisition occur or be announced, each right would, upon exercise, entitle a rights holder, other than the acquiring person and related persons, to purchase common shares at a 50% discount to the market price at the time.
Under the Rights Plan, a Permitted Bid is a bid made to all holders of the common shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding common shares, other than those owned by the offeror and certain related parties have been tendered, the offeror may take up and pay for the common shares but must extend the bid for a further 10 days to allow other shareholders to tender.
The issuance of common shares upon the exercise of the rights is subject to receipt of certain regulatory approvals.
Anti-takeover Laws
In Canada, takeover bids are governed by provincial corporate and securities laws and the rules of applicable stock exchanges. The following description of the rules relating to acquisitions of securities and takeover bids to which Canadian corporate and securities laws apply does not purport to be complete and is subject, and qualified in its entirety by reference, to applicable corporate and securities laws, which may vary from province to province.
A party (acquiror) who acquires beneficial ownership of, or control or direction over, more than 10% of the voting or equity securities of any class of a reporting issuer (or securities convertible into voting or equity securities of any class of a reporting issuer) will generally be required to file with applicable provincial regulatory authorities both a news release and a report containing the information prescribed by applicable securities laws. Subject to the below, the acquiror (including any party acting jointly or in concert with the acquiror) will be prohibited from purchasing any additional securities of the class of the target company previously acquired for a period commencing on the occurrence of an event triggering the aforementioned filing requirement and ending on the expiry of one business day following the filing of the report. This filing process and the associated restriction on further purchases also apply in respect of subsequent acquisitions of 2% or more of the securities of the same class (or securities convertible into voting or equity securities of any class of a reporting issuer). The restriction on further purchases does not apply to an acquiror that beneficially owns, or controls or directs, 20% or more of the outstanding securities of that class.
In addition to the foregoing, certain other Canadian legislation may limit a Canadian or non-Canadian entity’s ability to acquire control over or a significant interest in us, including the Competition Act (Canada) and the Investment Canada Act (Canada). Issuers may also approve and adopt shareholder rights plans or other defensive tactics designed to be triggered upon the commencement of an unsolicited bid and make the company a less desirable takeover target.
DESCRIPTION OF WARRANTS
The following summary of the general terms and provisions of the warrants represented by warrant agreements and warrant certificates that we may offer using this prospectus and a prospectus supplement is only a summary and does not purport to be complete. You must look at the applicable forms of warrant agreement and warrant certificate for a full understanding of the specific terms of any warrant. The forms of the warrant agreement and the warrant certificate will be filed or incorporated by reference as exhibits to the registration statement to which this prospectus is a part. See “Where You Can Find More Information” for information on how to obtain copies.
A prospectus supplement will describe the specific terms of the warrants offered under that prospectus supplement, including any of the terms in this section that will not apply to those warrants, and any special considerations, including tax considerations, applicable to investing in those warrants.
General
We may issue warrants to purchase common shares alone or together with other securities offered by the applicable prospectus supplement. The warrants may be issued independently or together with any securities and may be attached to or separate from the securities. We may enter into a warrant agreement with a warrant agent. If we elect to do so, the warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency or trust for or with any registered holders of warrants or beneficial owners of warrants.
The prospectus supplement relating to any warrants we offer will describe the specific terms relating to the offering. These terms may include some or all of the following:
|
● |
the offering price; |
|
● |
the currencies in which the warrants will be offered; |
|
● |
the total number of shares that may be purchased if all of the holders exercise the warrants; |
|
● |
the number of shares that may be purchased if a holder exercises any one warrant and the price at which and currencies in which shares may be purchased upon exercise; |
|
● |
the date on and after which the holder of the warrants can transfer them separately from the related underlying common shares; |
|
● |
the date on which the right to exercise the warrants begins and expires; |
|
● |
the triggering event and the terms upon which the exercise price and the number of underlying common shares that the warrants are exercisable into may be adjusted; |
|
● |
whether the warrants will be issued in registered or bearer form; |
|
● |
the identity of any warrant agent with respect to the warrants and the terms of the warrant agency agreement with that warrant agent; |
|
● |
a discussion of material U.S. federal income tax consequences; and |
|
● |
any other terms of the warrants. |
A holder of warrants may:
|
● |
exchange them for new warrants of different denominations; |
|
● |
present them for registration of transfer, if they are in registered form; and |
|
● |
exercise them at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement. |
Until the warrants are exercised, holders of the warrants will not have any of the rights of holders of the underlying common shares.
Exercise of Warrants
Each holder of a warrant is entitled to purchase the number of common shares at the exercise price described in the applicable prospectus supplement. After the close of business on the day when the right to exercise terminates (or a later date if we extend the time for exercise), unexercised warrants will become void.
Holders of warrants may exercise them by:
|
● |
delivering to the warrant agent the payment required to purchase the underlying common shares, as stated in the applicable prospectus supplement; |
|
● |
properly completing and signing the reverse side of their warrant certificate(s), if any, or other exercise documentation; and |
|
● |
delivering their warrant certificate(s), if any, or other exercise documentation to the warrant agent within the time specified by the applicable prospectus supplement. |
If you comply with the procedures described above, your warrants will be considered to have been exercised when the warrant agent receives payment of the exercise price. As soon as practicable after you have completed these procedures, we will issue and deliver to you the common shares that you purchased upon exercise. If you exercise fewer than all of the warrants represented by a warrant certificate, we will issue to you a new warrant certificate for the unexercised amount of warrants.
Amendments and Supplements to Warrant Agreements
We may amend or supplement a warrant agreement or warrant certificates without the consent of the holders of the warrants if the changes are not inconsistent with the provisions of the warrants and do not adversely affect the interests of the holders.
DESCRIPTION OF UNITS
We may, from time to time, issue units comprised of one or more of the other securities described in this prospectus in any combination. A prospectus supplement will describe the specific terms of the units offered under that prospectus supplement, and any special considerations, including tax considerations, applicable to investing in those units. You must look at the applicable prospectus supplement and any applicable unit agreement for a full understanding of the specific terms of any units. The form of unit agreement will be filed or incorporated by reference as an exhibit to the registration statement to which this prospectus is a part. See “Where You Can Find More Information” for information on how to obtain copies.
PLAN OF DISTRIBUTION
We may sell the securities from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination of these methods. We may sell the securities separately or together:
|
● |
through one or more underwriters or dealers in a public offering and sale by them; |
|
● |
through agents; and/or |
|
● |
directly to one or more purchasers. |
We may distribute the securities from time to time in one or more transactions:
|
● |
at a fixed price or prices, which may be changed; |
|
● |
at market prices prevailing at the time of sale; |
|
● |
at prices related to such prevailing market prices; or |
|
● |
at negotiated prices. |
We may solicit directly offers to purchase the respective securities being offered by this prospectus. We may also designate agents to solicit offers to purchase the respective securities from time to time. We will name in a prospectus supplement any agent involved in the offer or sale of our securities. If we utilize a dealer in the sale of the respective securities being offered by this prospectus, we will sell the respective securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. If we utilize an underwriter in the sale of the respective securities being offered by this prospectus, we will execute an underwriting agreement with the underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement that the underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we or the purchasers of securities for whom the underwriter may act as agent may compensate the underwriter in the form of underwriting discounts or commissions. In connection with the offering of securities, we may grant to the underwriters an option to purchase additional securities with an additional underwriting commission, as may be set forth in the accompanying prospectus supplement. If we grant any such option, the terms of such option will be set forth in the prospectus supplement for such securities. The underwriter may sell the securities to or through dealers, and the underwriter may compensate those dealers in the form of discounts, concessions or commissions. No Financial Industry Regulatory Authority (FINRA) member firm may receive compensation in excess of that allowable under FINRA rules, including Rule 5110, in connection with the offering of the securities.
We will provide in the applicable prospectus supplement any compensation we will pay to underwriters, dealers or agents in connection with the offering of the respective securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect thereof.
Our common shares are currently listed on The Nasdaq Capital Market. The other securities that may be offered under this prospectus and the related prospectus supplement may or may not be listed on a national securities exchange. To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. The transactions may be discontinued at any time.
We may authorize underwriters, dealers or agents to solicit offers by certain purchasers to purchase the respective securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. The contracts will be subject only to those conditions set forth in the prospectus supplement, and the prospectus supplement will set forth any commissions we pay for solicitation of these contracts.
We may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, the parties may sell securities covered by this prospectus and the applicable prospectus supplement, including short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be identified in the applicable prospectus supplement or a post-effective amendment to this registration statement. In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
The anticipated date of delivery of offered securities will be set forth in the applicable prospectus supplement relating to each offer.
LEGAL MATTERS
Unless the applicable prospectus supplement indicates otherwise, the validity of the securities in respect of which this prospectus is being delivered will be passed upon for us by Pushor Mitchell LLP, Kelowna, British Columbia, Canada, relating to matters of British Columbia or Canadian law, and Fox Rothschild LLP, New York, New York, relating to matters of New York law. Additional legal matters may be passed upon for us or any underwriters, dealers or agents by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements incorporated into this prospectus by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 have been audited by Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm. Their report, which is incorporated herein by reference, expresses an unqualified opinion on the consolidated financial statements. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at the SEC’s public reference room at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information about its public reference facilities and their copy charges.
We also file annual audited and interim unaudited financial statements, proxy statements and other information with the Ontario, Manitoba, Québec, Alberta and British Columbia Securities Commissions. Copies of these documents that are filed through the System for Electronic Document Analysis and Retrieval of the Canadian Securities Administrators are available at its website www.sedar.com.
In addition, we maintain a website that contains information regarding our company, including copies of reports, proxy statements and other information we file with the SEC. The address of our website is www.diamedica.com. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered by this prospectus. When used in this prospectus, the term “registration statement” includes amendments to the registration statement as well as the exhibits, schedules, financial statements and notes filed as part of the registration statement. This prospectus, which constitutes a part of the registration statement, does not contain all of the information in the registration statement. This prospectus omits information contained in the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and the common shares and other securities that may be offered by this prospectus, reference is made to the registration statement. Statements herein concerning the contents of any contract or other document are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed with the SEC as an exhibit to the registration statement, each such statement being qualified by and subject to such reference in all respects.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference the information we file with them. This allows us to disclose important information to you by referencing those filed documents. We have previously filed the documents set forth below with the SEC and are incorporating them by reference into this prospectus. Our SEC file no. is 001-36291.
|
● |
|
● |
|
● |
Quarterly Report on Form 10-Q for the quarter ended March 31, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended June 30, 2019; |
|
● |
Quarterly Report on Form 10-Q for the quarter ended September 30, 2019; |
|
● |
Current Reports on Form 8-K (only to the extent information is “filed” and not “furnished”) filed with the SEC on January 3, 2019, January 9, 2019, February 26, 2019, May 23, 2019, June 4, 2019, June 19, 2019, June 21, 2019, August 13, 2019, and October 30, 2019; and |
|
● |
the description of our common shares contained in our Amendment No. 1 to our registration statement on Form 8-A that we filed with the SEC on June 4, 2019, and any amendment or report filed for the purpose of updating this description. |
We also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is a part and before the effective date of the registration statement and after the date of this prospectus until the termination of the offering. The most recent information that we file with the SEC automatically updates and supersedes more dated information.
You may access our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statement, and amendments, if any, to those documents filed or furnished pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act with the SEC free of charge at the SEC’s website at www.sec.gov or our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
You can obtain a copy of any documents which are incorporated by reference in this prospectus or prospectus supplement, except for exhibits which are not specifically incorporated by reference into those documents, at no cost, by writing or telephoning us at:
DiaMedica Therapeutics Inc.
Two Carlson Parkway, Suite 260
Minneapolis, Minnesota 55447
Attention: Secretary
(763) 312-6755
Shares

Common Shares
____________________________
PROSPECTUS SUPPLEMENT
____________________________
Guggenheim Securities
Craig-Hallum Capital Group
____________________________
, 2020