UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________
FORM 10-K/A
(Amendment No. 1)
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2020
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________________ to __________________.
Commission file number: 001-36291
____________________
DIAMEDICA THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
____________________
|
British Columbia (State or other jurisdiction of incorporation or organization) |
Not Applicable (I.R.S. Employer Identification No.) |
|
Two Carlson Parkway, Suite 260 Minneapolis, Minnesota (Address of principal executive offices) |
55447 (Zip Code) |
Registrant’s telephone number, including area code: (763) 612-6755
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Voting Common Shares, no par value per share |
DMAC |
The Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☐ NO ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
Non-accelerated filer ☒ |
Smaller reporting company ☒ |
|
Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). YES ☐ NO ☒
The aggregate market value of the registrant’s voting common shares held by non-affiliates, computed by reference to the closing sales price at which the voting common shares were last sold as of June 30, 2020 (the last business day of the registrant’s most recently completed second fiscal quarter), as reported by The Nasdaq Capital Market on that date, was $78.5 million.
As of April 26, 2021, there were 18,786,157 voting common shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
EXPLANATORY NOTE
We filed our Annual Report on Form 10-K for the fiscal year ended December 31, 2020 (Original Filing) with the U.S. Securities and Exchange Commission (SEC) on March 10, 2021. We are filing this Amendment No. 1 (Amendment) on Form 10-K/A to amend the Original Filing solely for the purpose of including in Part III the information that was to be incorporated by reference from our definitive proxy statement for the 2021 annual general meeting of shareholders, because our definitive proxy statement will not be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2020. This information was previously omitted from the Original Filing in reliance on General Instruction G(3) to Form 10-K, which permits the information in the above-referenced items to be incorporated in the Form 10-K by reference from a definitive proxy statement involving the election of directors if such statement is filed no later than 120 days after our fiscal year end. This Amendment hereby amends and restates in their entirety the Form 10-K cover page and Items 10 through 14 of Part III.
In addition, pursuant to the rules and regulations promulgated by the SEC, we have also included in Part IV of this Amendment as exhibits currently dated certifications of our principal executive officer and principal financial officer as required under Section 302 of the Sarbanes-Oxley Act of 2002. Because no financial statements have been included in this Amendment and this Amendment does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4 and 5 of the certifications have been omitted. Additionally, we are not including the certificate required under Section 906 of the Sarbanes-Oxley Act of 2002 as no financial statements are being filed with this Amendment.
Except as described above, no other changes have been made to the Original Filing. Except as otherwise explicitly stated herein, this Amendment continues to speak as of the date of the Original Filing, and we have not updated the disclosures contained therein to reflect any events that occurred subsequent to the date of the Original Filing. The filing of this Amendment is not a representation that any statements contained in items of our Original Filing other than Items 10 through 14 of Part III and Part IV are true or complete as of any date subsequent to the Original Filing. This Amendment should be read in conjunction with our other filings made with the SEC subsequent to the date filed of the Original Filing, including any amendments to those filings, as well as our Current Reports on Form 8-K subsequent to the date of the Original Filing.
DIAMEDICA THERAPEUTICS INC.
AMENDMENT NO. 1 TO ANNUAL REPORT ON FORM 10-K/A
FISCAL YEAR ENDED DECEMBER 31, 2020
TABLE OF CONTENTS
| Page | ||
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 1 | |
| PART III | 2 | |
| Item 10. | Directors, Executive Officers and Corporate Governance | 2 |
| Item 11. | Executive Compensation | 15 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 30 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 33 |
| Item 14. | Principal Accounting Fees and Services | 35 |
| PART IV | 36 | |
| Item 15. | Exhibits and Financial Statement Schedules | 36 |
| SIGNATURES | 41 | |
_______________________
This Amendment contains certain forward-looking statements that are within the meaning of Section 27A of the United States Securities Act of 1933, as amended, and Section 21E of the United States Securities Exchange Act of 1934, as amended, and are subject to the safe harbor created by those sections. For more information, see “Cautionary Note Regarding Forward-Looking Statements.”
As used in this Amendment, references to “DiaMedica,” the “Company,” “we,” “our” or “us,” unless the context otherwise requires, refer to DiaMedica Therapeutics Inc. and its subsidiaries, all of which are consolidated in DiaMedica’s consolidated financial statements. References in this Amendment to “common shares” mean our voting common shares, no par value per share.
We own various unregistered trademarks and service marks, including our corporate logo. Solely for convenience, the trademarks and trade names in this Amendment are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the owner of such trademarks and trade names will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
[page intentionally left blank]
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this Amendment that are not descriptions of historical facts are forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 that are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and share price. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would,” the negative of these terms or other comparable terminology, and the use of future dates.
The forward-looking statements in this Amendment include, among other things, statements about:
|
● |
our plans to develop, obtain regulatory approval for and commercialize our DM199 product candidate for the treatment of acute ischemic stroke (AIS) and chronic kidney disease (CKD) and our expectations regarding the benefits of our DM199 product candidate; |
|
● |
our ability to conduct successful clinical testing of our DM199 product candidate for AIS and CKD and certain anticipated dates with respect to our pending and anticipated clinical trials; |
|
● |
our ability to obtain required regulatory approvals of our DM199 product candidate for AIS and CKD; |
|
● |
the perceived benefits of our DM199 product candidate over existing treatment options for AIS and CKD; |
|
● |
the potential size of the markets for our DM199 product candidate and our ability to serve those markets; |
|
● |
the rate and degree of market acceptance, both in the United States and internationally, of our DM199 product candidate for AIS and CKD; |
|
● |
our ability to partner with and generate revenue from biopharmaceutical or pharmaceutical partners to develop, obtain regulatory approval for and commercialize our DM199 product candidate for AIS and CKD; |
|
● |
the success, cost and timing of planned clinical trials, as well as our reliance on collaboration with third parties to conduct our clinical trials; |
|
● |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
● |
expectations regarding federal, state, and foreign regulatory requirements and developments, such as potential United States Food and Drug Administration (FDA) regulation of our DM199 product candidate for AIS and CKD; |
|
● |
expectations regarding competition and our ability to obtain data exclusivity for our DM199 product candidate for AIS and CKD; |
|
● |
our ability to obtain funding for our operations, including funding necessary to complete planned clinical trials and obtain regulatory approvals for our DM199 product candidate for AIS and CKD; |
|
● |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
● |
our expectations regarding our ability to obtain and maintain intellectual property protection for our DM199 product candidate; and |
|
● |
our anticipated use of the net proceeds from our 2020 public offerings. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under “Part I. Item 1A. Risk Factors in the Original Filing. Moreover, we operate in a very competitive and rapidly-changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Forward-looking statements should not be relied upon as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Except as required by law, including the securities laws of the United States, we do not intend to update any forward-looking statements to conform these statements to actual results or to changes in our expectations.
PART III
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
Directors and Executive Officers
The following table sets forth, as of April 26, 2021, the name, age and position of each current director and executive officer of our company:
|
Name |
Age |
Position |
||
|
Richard Pilnik(1)(2)(3)(4) |
64 |
Chairman of the Board |
||
|
Michael Giuffre, M.D.(1)(2)(3)(4) |
65 |
Director |
||
|
James Parsons(1)(2)(3)(4) |
55 |
Director |
||
|
Rick Pauls |
49 |
President and Chief Executive Officer, Director |
||
|
Scott Kellen |
55 |
Chief Financial Officer and Secretary |
||
|
Harry Alcorn, Pharm.D. |
65 |
Chief Medical Officer |
||
|
Sydney Gilman, Ph.D. |
68 |
Vice President, Regulatory Affairs |
(1) Independent Director
(2) Member of the Audit Committee
(3) Member of the Compensation Committee
(4) Member of the Nominating and Corporate Governance Committee
The principal occupations and recent employment history of each of our directors and executive officers are set forth below.
Additional Information about Current Directors and Executive Officers
The following paragraphs provide information about each current director and executive officer, including all positions held, principal occupation and business experience for the past five years, and the names of other publicly-held companies of which the director or executive officer currently serves as a director or has served as a director during the past five years. We believe that all of our directors display personal and professional integrity; satisfactory levels of education and/or business experience; broad-based business acumen; an appropriate level of understanding of our business and its industry and other industries relevant to our business; the ability and willingness to devote adequate time to the work of the Board of Directors and its committees; a fit of skills and personality with those of our other directors that helps build a board that is effective, collegial and responsive to the needs of our company; strategic thinking and a willingness to share ideas; a diversity of experiences, expertise and background; and the ability to represent the interests of all of our shareholders. The information presented below regarding each director also sets forth specific experience, qualifications, attributes and skills that led the Board of Directors to the conclusion that such individual should serve as a director in light of our business and structure.
Richard Pilnik has served as a member of the Board of Directors since May 2009. Mr. Pilnik serves as our Chairman of the Board. Mr. Pilnik has served as the President and member of the board of directors of Vigor Medical Services, Inc., a medical device company, since May 2017. From December 2015 to November 2017, he served as a member of the board of directors of Chiltern International Limited, a private leading mid-tier Clinical Research Organization, and was Chairman of the Board from April 2016 to November 2017. Mr. Pilnik has a 30-year career in healthcare at Eli Lilly and Company, a pharmaceutical company, and Quintiles Transnational Corp., a global pioneer in pharmaceutical services. From April 2009 to June 2014, he served as Executive Vice President and President of Quintiles Commercial Solutions, an outsourcing business to over 70 pharma and biotech companies. Prior to that, he spent 25 years at Eli Lilly and Company where he held several leadership positions, most recently as Group Vice President and Chief Marketing Officer from May 2006 to July 2008. He was directly responsible for commercial strategy, market research, new product planning and the medical marketing interaction. From December 2000 to May 2006, Mr. Pilnik served as President of Eli Lilly Europe, Middle East and Africa and the Commonwealth of Independent States, a regional organization of former Soviet Republics, and oversaw 50 countries and positioned Eli Lilly as the fastest growing pharmaceutical company in the region. Mr. Pilnik also held several marketing and sales management positions in the United States, Europe and Latin America. Mr. Pilnik currently serves on the board of directors of Vigor Medical Systems, Inc., NuSirt, an early-stage biopharma, and BIAL Farma, a Portuguese pharmaceutical company. Mr. Pilnik is an Emeritus Board Member of Duke University Fuqua School of Business. Mr. Pilnik previously served on the board of directors of Elan Pharmaceuticals, Chiltern International, the largest mid-size Clinical Research Organization, and Certara, L.P., a private biotech company focused on drug development modeling and biosimulation. Mr. Pilnik holds a Bachelor of Arts in Economics from Duke University and an MBA from the Kellogg School of Management at Northwestern University. Mr. Pilnik is a resident of Florida, USA.
We believe that Mr. Pilnik’s deep experience in the industry and his history and knowledge of our company enable him to make valuable contributions to the Board of Directors.
Michael Giuffre, M.D. has served as a member of the Board of Directors since August 2010. Since July 2009, Dr. Giuffre has served as a Clinical Professor of Cardiac Sciences and Pediatrics at the University of Calgary and has had an extensive portfolio of clinical practice, cardiovascular research and university teaching. Dr. Giuffre is actively involved in health care delivery, medical leadership and in the biotechnology business sector. From 2012 to October 2019, Dr. Giuffre served as Chief Scientific Officer and President of FoodChek Laboratory, a global developer and provider of proprietary rapid and accurate food safety tests for the detection of foodborne and environmental pathogens and other microorganisms, and also as a member of the board of directors of FoodChek Systems Inc. From November 2017 to October 2019, he served as FoodChek Systems Inc.'s Chairman of the Board. Dr. Giuffre previously served on the board of directors of the Canadian Medical Association (CMA), UNICEF Canada, the Alberta Medical Association (AMA), Can-Cal Resources Ltd, Vacci-Test Corporation, IC2E International Inc., MedMira Inc. and Brightsquid Dental, Inc. Dr. Giuffre has received a Certified and Registered Appointment and a Distinguished Fellow appointment by the American Academy of Cardiology. In 2005, he was awarded Physician of the Year by the Calgary Medical Society and in 2017 was "Mentor of the Year" for the Royal College of Physicians and Surgeons of Canada. Dr. Giuffre was also a former President of the AMA and the Calgary and Area Physicians Association and also a past representative to the board of the Calgary Health Region. Dr. Giuffre holds a Bachelor of Science in cellular and microbial biology, a Ph.D. candidacy in molecular virology, an M.D. and an M.B.A. He is Canadian Royal College board certified FRCPS in specialties that include Pediatrics and Pediatric Cardiology and has a subspecialty in Pediatric Cardiac Electrophysiology. Dr. Giuffre is currently a member of the board of directors of Avenue Living (AL) Asset Management, a private real estate company in Alberta, Canada and its affiliates, AL Real Estate Opportunity Trust and AgriSelect Trust. Dr. Giuffre is currently a resident of Alberta, Canada.
We believe that Dr. Giuffre’s medical experience, including as a practicing physician and professor, enable him to make valuable contributions to the Board of Directors.
James Parsons has served as a member of the Board of Directors since October 2015. Previously, Mr. Parsons served as our Vice President of Finance from October 2010 until May 2014. Since August 2011, Mr. Parsons has served as Chief Financial Officer and Corporate Secretary of Trillium Therapeutics Inc., a Nasdaq-listed immuno-oncology company. Mr. Parsons serves as a member of the board of directors and audit committee chair of Sernova Corp., which is listed on the TSX Venture Exchange. Mr. Parsons has been a Chief Financial Officer in the life sciences industry since 2000 with experience in therapeutics, diagnostics and devices. Mr. Parsons has a Master of Accounting degree from the University of Waterloo and is a Chartered Professional Accountant and Chartered Accountant. Mr. Parsons is a resident of Ontario, Canada.
We believe that Mr. Parsons’ financial experience, including his history and knowledge of our company, enable him to make valuable contributions to the Board of Directors.
Rick Pauls was appointed our President and Chief Executive Officer in January 2010. Mr. Pauls has served as a member of the Board of Directors since April 2005 and the Chairman of the Board from April 2008 to July 2014. Prior to joining DiaMedica, Mr. Pauls was the Co-Founder and Managing Director of CentreStone Ventures Inc., a life sciences venture capital fund, from February 2002 until January 2010. Mr. Pauls was an analyst for Centara Corporation, another early stage venture capital fund, from January 2000 until January 2002. From June 1997 until November 1999, Mr. Pauls worked for General Motors Acceptation Corporation specializing in asset-backed securitization and structured finance. Mr. Pauls previously served as an independent member of the board of directors of LED Medical Diagnostics, Inc. Mr. Pauls received his Bachelor of Arts in Economics from the University of Manitoba and his M.B.A. in Finance from the University of North Dakota. Mr. Pauls is a resident of Minnesota, USA.
We believe that Mr. Pauls’ experience in the biopharmaceutical industry as an executive and investor and his extensive knowledge of all aspects of our company, business, industry, and day-to-day operations as a result of his role as our President and Chief Executive Officer enable him to make valuable contributions to the Board of Directors. In addition, as a result of his role as President and Chief Executive Officer, Mr. Pauls provides unique insight into our future strategies, opportunities and challenges, and serves as the unifying element between the leadership and strategic direction provided by the Board of Directors and the implementation of our business strategies by management.
Scott Kellen joined DiaMedica as our Vice President of Finance in January 2018 and was appointed our Chief Financial Officer and Secretary in April 2018. Prior to joining DiaMedica, Mr. Kellen served as Vice President and Chief Financial Officer of Panbela Therapeutics, Inc., formerly known as Sun BioPharma, Inc., a publicly-traded clinical stage drug development company, from October 2015 until April 2018. From February 2010 to September 2015, Mr. Kellen served as Chief Financial Officer and Secretary of Kips Bay Medical, Inc., a publicly-traded medical device company, and became Chief Operating Officer of Kips Bay in March 2012. From November 2007 to May 2009, Mr. Kellen served as Finance Director of Transoma Medical, Inc. From 2005 to October 2007, Mr. Kellen served as Corporate Controller of ev3 Inc. From March 2003 to April 2005, Mr. Kellen served as Senior Manager, Audit and Advisory Services of Deloitte & Touche, LLP. Altogether, Mr. Kellen has spent more than 25 years in the life sciences industry, focusing on publicly traded early stage and growth companies. Mr. Kellen has a Bachelor of Science degree in Business Administration from the University of South Dakota and is a Certified Public Accountant (inactive).
Harry Alcorn Jr. Pharm.D. was appointed our Chief Medical Officer in August 2018. Prior to joining DiaMedica, Dr. Alcorn served as Chief Scientific Officer at DaVita Clinical Research (DCR), a company that provides clinical research services for Pharmaceutical and Biotech companies, from October 1997 to June 2018. While at DCR, Dr. Alcorn was responsible for clinical research operations, including the formation and management of the early clinical and late phase research services. Dr. Alcorn also founded the U.S. Renal Network, the first network of Phase 1 renal research sites in the United States. Dr. Alcorn developed DCR’s site management organization for clinical trials. Dr. Alcorn also served as an Executive Director, a Pharmacist and an Investigator at DCR. During this time, from January 2013 to December 2014, he also served on the Board of Directors for the Association of Clinical Pharmacology Units, an association of Phase 1 clinical trial sites. Dr. Alcorn has over 30 years of clinical research experience working with biotech and pharmaceutical companies, both public and private, in conducting research in renal, hepatic and cardiovascular disease. Dr. Alcorn has written and consulted on the development of several protocols and has served as Principal Investigator or Sub Investigator in numerous studies and, for several of these studies, presented study design and results to the FDA. Currently he holds clinical faculty appointments with the University of Minnesota, Creighton University, University of Nebraska Medical Center, Virginia Commonwealth and the University of Colorado, Denver. Dr. Alcorn graduated from Creighton University with a Bachelor of Pharmacy and went on to earn his Doctor of Pharmacy degree from University of Nebraska Medical Center.
Sydney A. Gilman, Ph.D. was appointed our Vice President, Regulatory Affairs in November 2019. Dr. Gilman is currently the founder and President of Trident Rx Consulting Services LLC, a regulatory consulting firm, a position he has held since January 2004. Dr. Gilman is a former FDA Chemistry reviewer. He spent six years at the FDA in various CDER Therapeutic Drug Divisions of the Center for Drug Evaluation and Research with consulting ties to both Biologics and Devices. Dr. Gilman also has an additional 20 years of experience in the pharmaceutical industry in positions ranging from Senior Scientist to Director to Vice President responsibilities. He earned his Bachelor of Science from Loyola College and a Ph.D. in Organic Chemistry from the University of Pittsburgh.
Management by Board of Directors
The Board of Directors is responsible for overseeing the management of DiaMedica and for the conduct of our affairs generally. Each director is elected annually by the shareholders and serves for a term that will end at the next annual general meeting of shareholders.
The Board of Directors facilitates its exercise of independent supervision over the management of DiaMedica through a combination of formal meetings of the Board of Directors and informal discussions amongst Board members. Due to the small size of the Board of Directors, and with a majority of independent directors, the Board of Directors manages governance matters both directly and through its Board committees, which are described in more detail below. The Board of Directors looks to management of DiaMedica to keep it apprised of all significant developments affecting the company and its operations. All major acquisitions, dispositions, investments, contracts and other significant matters outside the ordinary course of our business are subject to approval by the Board of Directors.
Corporate Governance Guidelines
The Board of Directors has established Corporate Governance Guidelines that describes our basic approach to corporate governance. A copy of these Corporate Governance Guidelines can be found on the ““Investor Relations—Governance” section of our corporate website www.diamedica.com. Among the topics addressed in our Corporate Governance Guidelines are:
|
● |
Board size and qualifications; |
|
● |
Selection of directors; |
|
● |
Board leadership; |
|
● |
Board committees; |
|
● |
Board and committee meetings; |
|
● |
Executive sessions of independent directors; |
|
● |
Meeting attendance by directors and non-directors; |
|
● |
Appropriate information and access; |
|
● |
Ability to retain advisors; |
|
● |
Conflicts of interest and director independence; |
|
● |
Board interaction with corporate constituencies; |
|
● |
Change of principal occupation; |
|
● |
Term limits; |
|
● |
Retirement and resignation policy; |
|
● |
Board compensation; |
|
● |
Stock ownership by directors; |
|
● |
Loans to directors and executive officers; |
|
● |
CEO evaluation; |
|
● |
Board and committee evaluation; |
|
● |
Succession planning; and |
|
● |
Communications with directors. |
Board Leadership Structure
Under our Corporate Governance Guidelines, the Board of Directors may select from its members a Chairman of the Board. The office of Chairman of the Board and the office of President and Chief Executive Officer may be held by one person. The Board of Directors believes it is best not to have a fixed policy on this issue and that it should be free to make this determination based on what it believes is best in light of current circumstances. The Board of Directors, acting as a group or through the Nominating and Corporate Governance Committee, will periodically review the leadership structure of the Board of Directors to assess whether it is appropriate given the specific characteristics and circumstances of DiaMedica. However, the Board of Directors does strongly endorse the concept of independent directors being in a position of leadership. If at any time, the Chief Executive Officer and Chairman of the Board are the same, the Board of Directors shall elect an independent director to serve as the lead director. The lead director will have the following duties and responsibilities in addition to such other duties and responsibilities as may be determined by the Board of Directors from time to time.
|
● |
chairing the executive sessions of the independent directors and calling meetings of the independent directors; |
|
● |
determining the agenda for the executive sessions of the independent directors and participating with the Chairman of the Board in establishing the agenda for Board meetings; |
|
● |
coordinating feedback among the independent directors and the Chief Executive Officer; |
|
● |
overseeing the development of appropriate responses to communications from shareholders and other interested persons addressed to the independent directors as a group; |
|
● |
on behalf of the independent directors, retaining legal counsel or other advisors as they deem appropriate in the conduct of their duties and responsibilities; and |
|
● |
performing such other duties as the Board of Directors deems appropriate from time to time. |
Mr. Pilnik currently serves as Chairman of the Board and Rick Pauls currently serves as President and Chief Executive Officer.
We currently believe this leadership structure is in the best interests of DiaMedica and our shareholders and strikes the appropriate balance between the President and Chief Executive Officer’s responsibility for the strategic direction, day-to day-leadership and performance of our company and the Chairman of our Board’s responsibility to guide overall strategic direction of our company and provide oversight of our corporate governance and guidance to our President and Chief Executive Officer and to set the agenda for and preside over board meetings. We recognize that different leadership structures may be appropriate for companies in different situations and believe that no one structure is suitable for all companies. We believe that our company is well-served by this leadership structure. We anticipate that the Board of Directors will periodically review our leadership structure and may make such changes in the future as it deems appropriate.
Under our Corporate Governance Guidelines, our independent directors will meet with no company management present during a portion of or after Board meetings on a regular basis but not less than two times per year. After each such executive session, and as otherwise necessary, our Chairman of the Board provides our Chief Executive Officer with any actionable feedback from our independent directors. The Board of Directors met five times in executive session during the fiscal year ended December 31, 2020.
Director Independence
The Board of Directors has affirmatively determined that three of DiaMedica’s current four directors are “independent directors” under the Nasdaq Listing Rules: Richard Pilnik, Michael Giuffre, M.D. and James Parsons. In making these affirmative determinations that such individuals are “independent directors,” the Board of Directors reviewed and discussed information provided by the directors and by DiaMedica with regard to each director’s business and personal activities as they may relate to DiaMedica and our management.
Board Committees
The Board of Directors has a standing Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee. Each of these committees has the composition described in the table below and the responsibilities described in the sections below. The Board of Directors has adopted a written charter for each committee of the Board of Directors which can be found on the ““Investor Relations—Governance” section of our corporate website www.diamedica.com. The Board of Directors from time to time may establish other committees.
The following table summarizes the current membership of each of our three board committees.
|
Director |
Audit Committee |
Compensation Committee |
Nominating and Corporate Governance Committee |
||||
|
Rick Pauls |
— |
— |
— |
||||
|
Michael Giuffre, M.D. |
√ |
Chair |
√ |
||||
|
James Parsons |
Chair |
√ |
√ |
||||
|
Richard Pilnik |
√ |
√ |
Chair |
Audit Committee
Responsibilities. The Audit Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to our annual and quarterly financial statements filed with the SEC and any applicable securities regulatory authorities of the provinces and territories of Canada, our financial reporting process, our internal control over financial accounting and disclosure controls and procedures, the annual independent audit of our financial statements and the effectiveness of our legal compliance and ethics programs. The Audit Committee’s primary responsibilities include:
|
● |
overseeing our financial reporting process, internal control over financial reporting and disclosure controls and procedures on behalf of the Board of Directors; |
|
● |
having sole authority to appoint, oversee, evaluate, retain and terminate the engagement of our independent registered public accounting firm and establish the compensation to be paid to the firm; |
|
● |
reviewing and pre-approving all audit services and permissible non-audit services to be provided to us by our independent registered public accounting firm; |
|
● |
establishing procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters and for the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters; and |
|
● |
overseeing our systems to monitor legal and ethical compliance programs, including the establishment and administration of (including the grant of any waiver from) a written code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. |
The Audit Committee has the authority to engage the services of outside experts and advisors as it deems necessary or appropriate to carry out its duties and responsibilities.
Composition. The current members of the Audit Committee are Dr. Giuffre, Mr. Parsons and Mr. Pilnik. Mr. Parsons is the Chair of the Audit Committee.
Each member of the Audit Committee qualifies as “independent” for purposes of membership on audit committees pursuant to the Nasdaq Listing Rules and the rules and regulations of the SEC and is “financially literate” as required by the Nasdaq Listing Rules. In addition, the Board of Directors has determined that Mr. Parsons qualifies as an “audit committee financial expert” as defined by the rules and regulations of the SEC and meets the qualifications of “financial sophistication” under the Nasdaq Listing Rules as a result of his extensive financial background and various financial positions he has held throughout his career. Shareholders should understand that these designations related to our Audit Committee members’ experience and understanding with respect to certain accounting and auditing matters do not impose upon any of them any duties, obligations or liabilities that are greater than those generally imposed on a member of the Audit Committee or of the Board of Directors.
Audit Committee Report. This report is furnished by the Audit Committee of the Board of Directors with respect to DiaMedica’s consolidated financial statements for the year ended December 31, 2020.
One of the purposes of the Audit Committee is to oversee DiaMedica’s accounting and financial reporting processes and the audit of DiaMedica’s annual consolidated financial statements. DiaMedica’s management is responsible for the preparation and presentation of complete and accurate financial statements. DiaMedica’s independent registered public accounting firm, Baker Tilly US, LLP, is responsible for performing an independent audit of DiaMedica’s annual consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States) and for issuing a report on their audit.
In performing its oversight role, the Audit Committee has reviewed and discussed DiaMedica’s audited consolidated financial statements for the year ended December 31, 2020 with DiaMedica’s management. Management represented to the Audit Committee that DiaMedica’s financial statements were prepared in accordance with generally accepted accounting principles. The Audit Committee has discussed with Baker Tilly US, LLP the matters required to be discussed under Public Company Accounting Oversight Board standards. The Audit Committee has received the written disclosures and the letter from Baker Tilly US, LLP required by applicable requirements of the Public Company Accounting Oversight Board regarding Baker Tilly US, LLP’s communications with the Audit Committee concerning independence. The Audit Committee has discussed with Baker Tilly US, LLP its independence and concluded that the independent registered public accounting firm is independent from DiaMedica and DiaMedica’s management.
Based on the review and discussions of the Audit Committee described above, in reliance on the unqualified opinion of Baker Tilly US, LLP regarding DiaMedica’s audited consolidated financial statements, and subject to the limitations on the role and responsibilities of the Audit Committee discussed above and in the Audit Committee’s charter, the Audit Committee recommended to the Board of Directors that DiaMedica’s audited consolidated financial statements for the fiscal year ended December 31, 2020 be included in its Annual Report on Form 10-K for the year ended December 31, 2020 for filing with the Securities and Exchange Commission.
This report is dated as of March 8, 2021.
Audit Committee
James Parsons, Chair
Michael Giuffre, M.D.
Richard Pilnik
Compensation Committee
Responsibilities. The Compensation Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to compensation of our Chief Executive Officer and other executive officers and administers our equity compensation plans. The Compensation Committee’s primary responsibilities include:
|
● |
determining all compensation for our Chief Executive Officer and other executive officers; |
|
● |
administering our equity-based compensation plans; |
|
● |
reviewing, assessing and approving overall strategies for attracting, developing, retaining and motivating our management and employees; |
|
● |
overseeing the development and implementation of succession plans for our Chief Executive Officer and other key executive officers and employees; |
|
● |
reviewing, assessing and approving overall compensation structure on an annual basis; and |
|
● |
recommending and leading a process for the determination of non-employee director compensation. |
The Compensation Committee has the authority to engage the services of outside experts and advisors as it deems necessary or appropriate to carry out its duties and responsibilities, and prior to doing so, assesses the independence of such experts and advisors from management.
Composition. The current members of the Compensation Committee are Dr. Giuffre, Mr. Parsons and Mr. Pilnik. Dr. Giuffre is the Chair of the Compensation Committee. The Board of Directors has determined that each of the members of the Compensation Committee is an “independent director” under the Nasdaq Listing Rules, a “non-employee director” within the meaning of Rule 16b-3 under the Exchange Act, and otherwise independent under the rules and regulations of the SEC.
Processes and Procedures for Consideration and Determination of Executive Compensation. As described in more detail above under “—Responsibilities,” the Board of Directors has delegated to the Compensation Committee the responsibility, among other things, to determine any and all compensation payable to our executive officers, including annual salaries, incentive compensation, long-term incentive compensation, perquisites and any and all other compensation, and to administer our equity-based compensation plans. The Compensation Committee has the full power and authority of the Board of Directors to perform these duties and to fulfill these responsibilities. Under the terms of its formal written charter, the Compensation Committee has the power and authority, to the extent permitted by applicable law, to delegate all or a portion of its duties and responsibilities to a subcommittee of the Compensation Committee. Except for a delegation of authority to the Company’s Chief Executive Officer and Chief Financial Officer to approve equity compensation awards under the Company’s shareholder-approved plan to newly-hired employees other than the executive officers of the Company, the Compensation Committee has not delegated any of its duties and responsibilities to subcommittees, but rather has taken such actions as a committee, as a whole.
In 2018, the Compensation Committee engaged the services of 21-Group, an independent compensation consultant, to assist the Compensation Committee in developing a comprehensive compensation strategy based upon compensation levels at benchmark companies for DiaMedica. The Compensation Committee used the information in this report, recommendations from the 21-Group and discussions with management, to establish a compensation strategy and set target compensation levels for officers and non-employee directors. In making final decisions regarding compensation to be paid to our executive officers, the Compensation Committee considers several factors, including the benchmarking information gathered by its compensation consultant, the achievement by DiaMedica of pre-established performance objectives, the general performance of DiaMedica and the individual officers, the performance of DiaMedica and other factors that may be relevant. The Compensation Committee retained 21-Group again in 2020 and in the beginning of 2021 to update its executive officer and non-employee director compensation analyses.
Final deliberations and decisions by the Compensation Committee regarding the form and amount of compensation to be paid to our executive officers are made by the Compensation Committee, without the presence of any executive officer of our company.
Processes and Procedures for Consideration and Determination of Director Compensation. As mentioned above under “—Responsibilities,” the Board of Directors has delegated to the Compensation Committee the responsibility, among other things, to review and make recommendations to the Board of Directors concerning compensation for non-employee members of the Board of Directors, including but not limited to retainers, meeting fees, committee chair and member retainers and equity compensation. Decisions regarding director compensation made by the Compensation Committee are not considered final and are subject to final review and approval by the entire Board of Directors. In making recommendations to the Board of Directors regarding compensation to be paid to our non-employee directors, the Compensation Committee considers fees and other compensation paid to directors of benchmark companies as gathered by its compensation consultant, the number of board and committee meetings that our directors are expected to attend and other factors that may be relevant. In making final decisions regarding non-employee director compensation, the Board of Directors considers the same factors and the recommendation of the Compensation Committee.
Nominating and Corporate Governance Committee
Responsibilities. The Nominating and Corporate Governance Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to director nominations and corporate governance. The primary responsibilities of the Nominating and Corporate Governance Committee include:
|
● |
identifying individuals qualified to become members of the Board of Directors, which includes reviewing and considering director nominees submitted by shareholders; |
|
● |
recommending director nominees for each annual general meeting of our shareholders and director nominees to fill any vacancies that may occur between general meetings of shareholders; |
|
● |
being aware of best practices in corporate governance matters and developing and recommending to the Board of Directors a set of corporate governance guidelines to govern the Board of Directors, its committees, DiaMedica and our employees; |
|
● |
recommending director diversity, retirement age, tenure and refreshment policies; |
|
● |
developing and overseeing an orientation process for new directors; and |
|
● |
developing and overseeing a periodic Board of Directors and Board committee evaluation process. |
The Nominating and Corporate Governance Committee has the authority to engage the services of outside experts and advisors as it deems necessary or appropriate to carry out its duties and responsibilities.
Orientation and Continuing Education of Directors. The Nominating and Corporate Governance Committee is responsible for developing and overseeing an orientation process for all new members of the Board of Directors. New directors are provided with access to our recent, publicly filed documents, technical reports and internal financial information and given copies of all Board of Director minutes and corporate governance materials. Directors are encouraged to ask questions and communicate with management, auditors and technical consultants to keep themselves current with industry trends and developments and changes in legislation. Continuing education is an important compliance requirement to promote the competence and integrity of Board members. Our directors are encouraged to take part in relevant education programs offered by appropriate regulatory bodies.
Composition. The current members of the Nominating and Corporate Governance Committee are Dr. Giuffre, Mr. Parsons and Mr. Pilnik. Mr. Pilnik is the Chair of the Nominating and Corporate Governance Committee. The Board of Directors has determined that each of the members of the Nominating and Corporate Governance Committee is an “independent director” under the Nasdaq Listing Rules.
Director Qualifications and the Nomination Process
The Board of Directors seeks to ensure that the Board is composed of members whose particular experience, qualifications, attributes and skills, when taken together, will allow the Board to satisfy its oversight responsibilities effectively. New directors will be approved by the Board after evaluation and recommendation by the Nominating and Corporate Governance Committee. In identifying candidates for director, the Nominating and Corporate Governance Committee and the Board take into account the following:
|
● |
the comments and recommendations of Board members regarding the qualifications and effectiveness of the existing Board, or additional qualifications that may be required when selecting new Board members; |
|
● |
the requisite expertise and sufficiently diverse backgrounds of the Board’s overall membership composition; |
|
● |
the independence of outside directors and other possible conflicts of interest of existing and potential members of the Board; and |
|
● |
any other factors they consider appropriate. |
When considering directors and nominees the Nominating and Corporate Governance Committee and the Board of Directors focuses primarily on the information discussed in each of the directors’ individual biographies.
The Nominating and Corporate Governance Committee will consider director candidates recommended to it by our shareholders. Those candidates must be qualified and exhibit the experience and expertise required of the Board’s own pool of candidates, as well as have an interest in our business and demonstrate the ability to attend and prepare for Board, committee, and shareholder meetings. Any candidate must provide a written statement, in advance, affirming his or her willingness and interest in serving on the Board. Candidates should represent the interests of all shareholders and not those of a special interest group. The Nominating and Corporate Governance Committee will evaluate candidates recommended by shareholders using the same criteria it uses to evaluate candidates recommended by others as described above. A shareholder that desires to nominate a person for election to the Board of Directors at a meeting of shareholders must follow the specified advance notice requirements contained in, and provide the specific information required by the BCBCA. During the fourth quarter of fiscal 2020, we made no material changes to the procedures by which shareholders may recommend nominees to our Board of Directors.
Board Diversity
The Nominating and Corporate Governance Committee is responsible for reviewing with the Board of Directors, on an annual basis, the appropriate characteristics, skills and experience required for the Board of Directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the Nominating and Corporate Governance Committee, in recommending candidates for election, and the Board of Directors in approving (and, in the case of vacancies, appointing) such candidates, take into account many factors, including the following:
|
● |
personal and professional integrity, ethics and values; |
|
● |
experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
|
● |
strong finance experience; |
|
● |
relevant social policy concerns; |
|
● |
experience relevant to our industry; |
|
● |
experience as a board member or executive officer of another publicly held company; |
|
● |
relevant academic expertise or other proficiency in an area of our operations; |
|
● |
diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
|
● |
diversity of background and perspective, including, but not limited to, with respect to age, gender, race, place of residence and specialized experience; |
|
● |
practical and mature business judgment, including, but not limited to, the ability to make independent analytical inquiries; and |
|
● |
any other relevant qualifications, attributes or skills. |
The Board of Directors evaluates each individual in the context of the Board of Directors as a whole, with the objective of assembling a group that can best perpetuate the success of the business and represent shareholder interests through the exercise of sound judgment using its diversity of experience in these various areas. In determining whether to recommend a director for re-election, the Nominating and Corporate Governance Committee may also consider the director’s past attendance at meetings and participation in and contributions to the activities of the Board of Directors.
We believe that a board of directors made up of highly qualified individuals from diverse backgrounds promotes better corporate governance, performance and effective decision-making. Our Board of Directors has not, at this time, adopted any fixed policies, targets or quotas relating to the representation on the Board of Directors or in executive officer positions based upon any external physiological attribute, including gender, as we do not believe that quotas or a formulaic approach necessarily result in the identification or selection of the best candidates. The Nominating and Corporate Governance Committee nonetheless makes efforts to ensure that directors and officers have a wide range of skills, experiences and backgrounds to meet our needs. To support this objective, the Nominating and Corporate Governance Committee will, when seeking candidates for Board of Directors or executive positions, among other things, (a) consider candidates who are highly qualified based on their experience, functional expertise and personal skills and qualities; and (b) consider diversity criteria including gender and geographical background of the candidate. As of the date of this report, no women (0%) are on our Board of Directors or are executive officers of DiaMedica.
Role of Board in Risk Oversight Process
Risk is inherent with every business. We face a number of risks, including regulatory, compliance, legal, competitive, financial (accounting, credit, interest rate, liquidity and tax), operational, political, strategic and reputational risks. Our management is responsible for the day-to-day management of risks faced by us, while the Board of Directors, as a whole and through its committees, has responsibility for the oversight of risk management. In its risk oversight role, the Board of Directors ensures that the risk management processes designed and implemented by management are adequate and functioning as designed. The Board of Directors oversees risks through the establishment of policies and procedures that are designed to guide daily operations in a manner consistent with applicable laws, regulations and risks acceptable to us. Our President and Chief Executive Officer, who is also a member of the Board of Directors, regularly discusses with the Board of Directors the strategies and risks facing our company.
The standing committees of the Board of Directors oversee risks associated with their respective principal areas of focus. The Audit Committee’s role includes a particular focus on the qualitative aspects of financial reporting to shareholders and on our processes for the management of business and financial risk. The Audit Committee, along with management, is also responsible for developing and participating in a process for review of important financial and operating topics that present potential significant risk to our company. The Compensation Committee is responsible for overseeing risks and exposures associated with our compensation programs and arrangements, including our executive and director compensation programs and arrangements, and management succession planning. The Nominating and Corporate Governance Committee oversees risks relating to our corporate governance matters and policies and director succession planning.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics applicable to all of our directors, officers and employees, in accordance with Section 406 of the Sarbanes-Oxley Act of 2002, the rules of the SEC promulgated thereunder and the Nasdaq Listing Rules. We monitor employee and director compliance with our code of business conduct and ethics through employee and director reporting. Violations may be reported to supervisors, the Chief Financial Officer or, alternatively, to the Chair of the Audit Committee via e-mail. We investigate and discipline all reported violations as appropriate. In the event that any changes are made or any waivers from the provisions of the code of business conduct and ethics are made, these events would be disclosed on our website or in a Current Report on Form 8-K within four business days of such event. The code of business conduct and ethics is posted on our website at www.diamedica.com. Copies of the code of business conduct and ethics will be provided free of charge upon written request directed to Corporate Secretary, DiaMedica Therapeutics Inc., Two Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447.
Board and Committee Meetings and Attendance
The table below summarizes the attendance of each director for meetings of the Board of Directors and the meetings of all Board committees on which the director served during the fiscal year ended December 31, 2020:
|
Director |
Number of Board Meetings Attended |
Number of Audit Committee Meetings Attended(1) |
Number of Compensation Committee Meetings Attended |
Number of Nominating and Corporate Governance Committee Meetings Attended |
||||||||
|
Rick Pauls |
10 | N/A | N/A | N/A | ||||||||
|
Michael Giuffre, M.D. |
10 | 6 | 6 | 3 | ||||||||
|
James Parsons |
10 | 6 | 6 | 3 | ||||||||
|
Richard Pilnik |
10 | 6 | 6 | 3 | ||||||||
|
Total Meetings Held |
10 | 6 | 6 | 3 |
(1) The Audit Committee met twice in executive session with Baker Tilly US, LLP, our independent registered public accounting firm.
Policy Regarding Director Attendance at Annual General Meetings of Shareholders
Directors are encouraged, but not required, to attend our annual general meetings of shareholders. Messrs. Pauls, Pilnik and Parsons attended the 2020 Annual General Meeting of Shareholders either in person, by telephone or by video conference.
Complaint Procedures
The Audit Committee has established procedures for the receipt, retention and treatment of complaints received by DiaMedica regarding accounting, internal accounting controls or auditing matters. These procedures provide for the submission by our employees, on a confidential and anonymous basis, of concerns regarding questionable accounting or auditing matters. Our personnel with such concerns are encouraged to discuss their concerns with our compliance officer, outside legal counsel or Audit Committee Chair.
Process Regarding Shareholder Communications with Board of Directors
Shareholders may communicate with the Board of Directors or any one particular director by sending correspondence, addressed to DiaMedica’s Corporate Secretary, DiaMedica Therapeutics Inc., Two Carlson Parkway, Suite 260, Minneapolis, MN 55447 with an instruction to forward the communication to the Board of Directors or one or more particular directors. DiaMedica’s Corporate Secretary will promptly forward all such shareholder communications to the Board of Directors or the one or more particular directors, with the exception of any advertisements, solicitations for periodical or other subscriptions and other similar communications.
|
Item 11. |
Executive Compensation |
Executive Compensation Overview
This section addresses the compensation of our President and Chief Executive Officer and the two most highly compensated executive officers for the year ended December 31, 2020:
|
● |
Rick Pauls, our President and Chief Executive Officer; |
|
● |
Scott Kellen, our Chief Financial Officer and Corporate Secretary; and |
|
● |
Harry Alcorn, Jr., Pharm.D., our Chief Medical Officer. |
These executive officers are collectively referred to as the named executive officers.
When reading this Executive Compensation Overview, please note that we are an emerging growth company under the Jumpstart our Business Startups Act (JOBS Act) and are not required to provide a “Compensation Discussion and Analysis” of the type required by Item 402 of SEC Regulation S-K. This Executive Compensation Overview is intended to supplement the SEC-required disclosure, which is included in this section, and it is not a Compensation Discussion and Analysis.
Compensation Philosophy
The Compensation Committee generally targets executive compensation at the 50th percentile of our peer group as discussed below under “—Elements of Our Executive Compensation Program.”
Use of Market Data
We strive to compensate our executive officers competitively relative to other companies that are similar to us from a market capitalization, revenue, number of employees and clinical development perspective. To ensure reasonableness and competitiveness of our executive compensation packages relative to our peer companies, the Compensation Committee evaluates our peer group with the aid of our independent compensation consultant and with input from management. The peer group used to help determine 2020 compensation was prepared by our independent compensation consultant in 2018 and consisted of the following 12 other companies in the same industry and with similar characteristics from a market capitalization, revenue, number of employees and clinical development perspective.
|
Actinium Pharmaceuticals, Inc. |
Cidara Therapeutics, Inc. |
Regulus Therapeutics Inc. |
|
Aptose Biosciences Inc. |
CohBar, Inc. |
Sun BioPharma, Inc. |
|
Athersys, Inc. |
Oncolytics Biotech Inc. |
Trillium Therapeutics Inc. |
|
Cellectar Biosciences, Inc. |
Oncomed Pharmaceuticals, Inc. |
Zafgen, Inc. |
Data from this peer group, therefore, was considered in the compensation benchmarking process as one input in helping us determine appropriate pay levels.
Use of Consultants
The Compensation Committee has the authority to engage the services of outside experts and advisors as it deems necessary or appropriate to carry out its duties and responsibilities, and prior to doing so, assesses the independence of such experts and advisors from management. During 2018, the Compensation Committee engaged the 21-Group, a compensation consultant, to assist the Compensation Committee in designing and reviewing our executive compensation program. The Compensation Committee retained 21-Group again in 2020 and in the beginning of 2021 to update its executive officer and non-employee director compensation analyses. The 21-Group does not provide any services to our company other than those for which it has been retained by the Compensation Committee.
Elements of Our Executive Compensation Program
During 2020, our executive compensation program consisted of several key elements, which are described in the table below along with the key characteristics of, and the purpose for, each element. The following table also describes any key 2020 changes to each of these elements.
|
Element |
Key Characteristics |
Purpose |
Key 2020 Changes |
|||
|
Base Salary
(Fixed, Cash) |
A fixed amount, paid in cash periodically throughout the year and reviewed annually and, if appropriate, adjusted. |
Provides a source of fixed income that is market competitive and reflects |
Our named executive officers received increases of 3% over their respective 2019 base salaries. |
|||
|
Short-Term Incentive (STI)
(Variable, Cash) |
A variable, short-term element |
Motivates and rewards our executives for achievement |
Our named executive officers received STI cash payments equal to 95% of their respective target bonus opportunities based on the achievement of corporate performance and individual performance objectives. In determining achievement levels, the Compensation Committee took into consideration the COVID-19 pandemic and its effect on our business and management’s response to the pandemic, especially with respect to the continuation of our clinical trial work. |
|||
|
Long-Term Incentives (LTI)
(Variable, Equity-Based Awards) |
A variable, long-term element of compensation that is provided in the form of time-vested stock option awards. |
Aligns the interests of our executives with our shareholders; encourages our executives to focus on the Company’s long-term performance; promotes retention of our executives; and encourages significant ownership of our common shares. |
Our named executive officers received stock option awards, which vest quarterly over three years. |
|||
|
Retirement Benefits |
Includes a defined contribution retirement plan with a discretionary Company match. |
Provides an opportunity for employees to save and prepare financially for retirement. |
No changes. |
We describe each key element of our executive compensation program in more detail in the following pages, along with the compensation decisions made in 2020. The compensation paid to our named executive officers is governed, in part, by written employment agreements with them, which are described below under “—Employment Agreements.” The named executive officers also have termination and change in control benefits as set forth in their respective employment agreements. See “—Post-Termination Severance and Change in Control Arrangements.”
Pay for Performance and Pay Mix
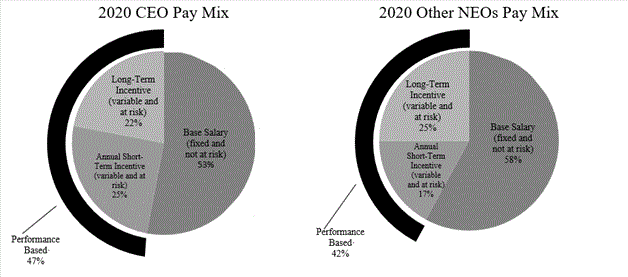
We seek to motivate management to achieve corporate objectives and increase shareholder value through incentive plans that reward higher performance with increased incentive payouts and hold management accountable for performance that falls below targeted levels by paying reduced or no incentive payouts. Accordingly, in general, our executive compensation program emphasizes variable, at-risk, pay elements as a significant portion of each executive’s total compensation package.
The breakdown of variable, at-risk, pay (broken out between short-term incentives and long-term incentives) compared to fixed pay (i.e., base salary) reported for 2020 in the Summary Compensation Table for our President and Chief Executive Officers and other named executive officers is as follows:

Base Salary
We provide a base salary for our named executive officers, which is not subject to company or individual performance risk. We recognize the need for most executives to receive at least a portion of their total compensation in the form of a guaranteed base salary that is paid in cash regularly throughout the year. The base salaries set for our named executive officers are intended to provide a steady income regardless of share price performance, allowing executives to focus on both near-term and long-term goals and objectives without undue reliance on short-term share price performance or market fluctuations.
We initially fix base salaries for our executives at a level that we believe enables us to hire and retain them in a competitive environment and to reward satisfactory individual performance and a satisfactory level of contribution to our overall business objectives. The Compensation Committee reviews and approves any increases in base salaries for our named executive officers.
The base salary for each of our named executive officers for fiscal 2020 compared to fiscal 2019 is as follows:
|
Name |
Fiscal 2020 |
Fiscal 2019 |
% Change from Fiscal 2019 |
|||||||||
|
Rick Pauls |
$ | 458,350 | $ | 445,000 | 3.0 | % | ||||||
|
Scott Kellen |
278,100 | 270,000 | 3.0 | % | ||||||||
|
Harry Alcorn, Jr., Pharm.D. |
293,550 | 285,000 | 3.0 | % | ||||||||
The base salary increases for each of our named executive officers were intended to provide for cost of living adjustments.
Annual Short-Term Incentive Compensation
In addition to base compensation, we provide our named executive officers the opportunity to earn short-term incentive (STI) compensation based on the achievement of certain annual corporate and individual performance goals. Our STI program directly aligns the interests of our executive officers and shareholders by providing an incentive for the achievement of key corporate and individual performance objectives that are critical to the success of our company and linking a significant portion of each executive’s annual compensation to the achievement of such objectives.
Under the 2020 STI program, each named executive officer had a target incentive percentage that was a percentage of their base salary:
|
Name |
Percentage of Salary Base |
|||
|
Rick Pauls |
50 | % | ||
|
Scott Kellen |
30 | % | ||
|
Harry Alcorn, Jr., Pharm.D. |
30 | % | ||
2020 STI payouts were based primarily on the achievement of three pre-established corporate performance objectives that related to clinical development milestones and either one or two individual performance objectives that related to each named executive’s corporate responsibilities. The STI payouts reflected adjustments in light of the COVID-19 pandemic and its effect on our business and management’s response to the pandemic, especially with respect to the continuation of our clinical trials, resulting in payouts representing 95% of each officer’s respective target bonus opportunity:
|
Officer Name and Position |
2020 Base Salary |
Target Incentive Percentage of Base Salary |
Target Bonus Opportunity |
2020 Actual Payout |
||||||||||||
|
Rick Pauls |
$ | 458,350 | 50 | % | $ | 229,175 | $ | 217,716 | ||||||||
|
Scott Kellen |
278,100 | 30 | % | 83,430 | 79,238 | |||||||||||
|
Harry Alcorn, Jr., Pharm.D. |
293,550 | 30 | % | 88,065 | 83,662 | |||||||||||
Long-Term Equity-Based Incentive Compensation
The long-term equity-based incentive compensation component consists of stock options granted under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan. Long-term equity-based incentives are intended to comprise a significant portion of each executive’s compensation package, consistent with our executive compensation objective to align the interests of our executives with the interests of our shareholders.
The Compensation Committee believes that options effectively incentivize executives to maximize company performance over the long-term, as the value of awards is directly tied to an appreciation in the value of our common shares. Stock options also provide an effective retention mechanism because of vesting provisions. An important objective of our long-term equity-based incentive program is to strengthen the relationship between the long-term value of our common shares and the potential financial gain for our executives. Stock options provide recipients with the opportunity to purchase our common shares at a price fixed on the grant date regardless of future market price. Because stock options become valuable only if the share price increases above the exercise price and the option holder remains employed during the period required for the option to vest, they provide an incentive for an executive to remain employed. In addition, stock options link a portion of an executive’s compensation to the interests of our shareholders by providing an incentive to achieve corporate goals and increase the market price of our common shares over time.
The table below sets forth the stock options that we granted to our named executive officers in 2020, which options vest quarterly over three years:
|
Name |
Grant Date |
Grant Date Fair Value |
Number of Shares Underlying Options |
Exercise Price |
||||||||||
|
Rick Pauls |
06/01/20 |
$ | 190,624 | 56,000 | $ | 4.64 | ||||||||
|
Scott Kellen |
06/01/20 |
119,140 | 35,000 | 4.64 | ||||||||||
|
Harry Alcorn, Jr., Pharm.D. |
06/01/20 |
119,140 | 35,000 | 4.64 | ||||||||||
All Other Compensation
It is generally our policy not to extend perquisites to our executives that are not available to our employees generally. Our executives receive benefits that are also received by our other employees, including participation in the DiaMedica USA, Inc. 401(k) Plan and health, dental, disability and life insurance benefits.
Employment Agreements
In September 2018, we entered into an employment agreement with each of our executive officers, which provides for an annual base salary, subject to periodic reviews, incentive based compensation, equity-based compensation and benefits, in each case as determined by the Board of Directors (or a committee thereof) from time to time. The agreements contain standard confidentiality, non-competition, non-solicitation and assignment of intellectual property provisions. The agreements also contains standard severance and change in control provisions which are described under “—Post-Termination Severance and Change in Control Arrangements.”
Post Termination Severance and Change in Control Arrangements
Severance Arrangements. Under the terms of the employment agreements with our executive officers, if we terminate the executive’s employment without “cause”, the executive will be entitled to: (i) salary continuation payments for 12 months in the case of Mr. Pauls and nine months in the case of each of the other executives, (ii) Consolidated Omnibus Budget Reconciliation Act (COBRA) premium reimbursement during the salary continuation period, (iii) a pro rata portion of their target annual bonus for the year of termination, and (iv) immediate acceleration of their equity awards. These severance benefits are subject to the executive executing a separation agreement and release of claims. “Cause” is defined in the employment agreements as: (i) gross negligence or willful failure to perform the executive’s duties and responsibilities to DiaMedica; (ii) commission of any act of fraud, theft, embezzlement, financial dishonesty or any other willful misconduct that has caused or is reasonably expected to result in injury to DiaMedica; (iii) conviction of, or pleading guilty or nolo contendere to, any felony or a lesser crime involving dishonesty or moral turpitude; (iv) material breach by the executive of any of their obligations under the agreement or any written agreement or covenant with DiaMedica, including the policies adopted from time to time by DiaMedica applicable to all executives, that has not been cured within 30 days of notice of such breach; or (v) we terminate the employment of the executive in connection with a liquidation, dissolution or winding down of DiaMedica. We believe that the form and amount of these severance benefits are fair and reasonable to both DiaMedica and our executives. The Compensation Committee reviews our severance arrangements periodically to ensure that they remain necessary and appropriate.
Change in Control Arrangements. To encourage continuity, stability and retention when considering the potential disruptive impact of an actual or potential corporate transaction, we have established change in control arrangements, including provisions in the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan (2019 Plan) and executive employment agreements. These arrangements are designed to incentivize our executives to remain with our company in the event of a change in control or potential change in control.
Under the terms of the 2019 Plan, subject to the terms of the applicable award agreement or an individual agreement between DiaMedica and a participant, upon a change in control, the Board of Directors may, in its discretion, determine whether some or all outstanding options and stock appreciation rights shall become exercisable in full or in part, whether the restriction period and performance period applicable to some or all outstanding restricted stock awards and restricted stock unit awards shall lapse in full or in part and whether the performance measures applicable to some or all outstanding awards shall be deemed to be satisfied. The Board of Directors may further require that shares of stock of the corporation resulting from such a change in control, or a parent corporation thereof, be substituted for some or all of our common shares subject to an outstanding award and that any outstanding awards, in whole or in part, be surrendered to us by the holder, to be immediately cancelled by us, in exchange for a cash payment, shares of capital stock of the corporation resulting from or succeeding us or a combination of both cash and such shares of stock.
Under the terms of the employment agreements that we entered into with our executives in September 2018, if we terminate the executive’s employment without “cause” or the executive terminates their employment with “good reason” in connection with or within 12 months after a “change in control,” the executive will be entitled to: (i) salary continuation payments for 18 months in the case of Mr. Pauls and 12 months in the case of each of the other executives, (ii) COBRA premium reimbursement during the salary continuation period, (iii) a pro rata portion of their target annual bonus for the year of termination, and (iv) immediate acceleration of their equity awards. These severance benefits are subject to the executive executing a separation agreement and release of claims.
“Good reason” is defined in the employment agreements as the executive’s resignation within 30 days following the expiration of any cure period following the occurrence of one or more of the following, without the executive’s express written consent: (i) a material reduction of the executive’s duties, authority, reporting level, or responsibilities, relative to their duties, authority, reporting level, or responsibilities in effect immediately prior to such change in control; (ii) a material reduction in the executive’s base compensation; or (iii) DiaMedica’s requiring of the executive to change the principal location at which the executive is to perform services by more than 50 miles.
“Change in control” is defined in the employment agreements as the occurrence of any of the following: (i) the acquisition, other than from us, by any individual, entity or group of beneficial ownership of 50% or more of either our then outstanding common shares or the combined voting power of our then outstanding voting securities entitled to vote generally in the election of directors; (ii) the consummation of a reorganization, merger or consolidation of DiaMedica, in each case, with respect to which all or substantially all of the individuals and entities who were the respective beneficial owners of our common shares and voting securities immediately prior to such reorganization, merger or consolidation do not, following such reorganization, merger or consolidation, beneficially own, directly or indirectly, more than 50% of, respectively, of then outstanding common shares and the combined voting power of then outstanding voting securities entitled to vote generally in the election of directors, as the case may be, of the corporation resulting from such reorganization, merger or consolidation; or (iii) the sale or other disposition of all or substantially all of our assets.
We believe these change in control arrangements are an important part of our executive compensation program in part because they mitigate some of the risk for executives working in a smaller company where there is a meaningful risk that DiaMedica may be acquired. Change in control benefits are intended to attract and retain qualified executives who, absent these arrangements and in anticipation of a possible change in control of our company, might consider seeking employment alternatives to be less risky than remaining with our company through the transaction. We believe that the form and amount of these change in control benefits are fair and reasonable to both our company and our executives. The Compensation Committee periodically reviews our change in control arrangements to ensure that they remain necessary and appropriate.
Indemnification Agreements
We have entered into indemnification agreements with all of our executive officers. The indemnification agreements are governed exclusively by and construed according to the substantive laws of the BCBCA, without regard to conflicts-of-laws principles that would require the application of any other law, and provide, among other things, for indemnification, to the fullest extent permitted by law and our Articles, against any and all expenses (including attorneys’ fees) and liabilities, judgments, fines and amounts paid in settlement that are paid or incurred by the executive or on his or her behalf in connection with such action, suit or proceeding. We will be obligated to pay these amounts only if the executive acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of our company and, in the case of a criminal or administrative proceeding that is enforced by a monetary penalty, he or she had reasonable grounds for believing that his or her conduct was lawful. The indemnification agreements provide that the executive will not be indemnified and expenses advanced with respect to an action, suit or proceeding initiated by the executive unless (i) so authorized or consented to by the Board of Directors or DiaMedica has joined in such action, suit or proceeding or (ii) the action, suit or proceeding is one to enforce the executive’s rights under the indemnification agreement. Our indemnification and expense advance obligations are subject to the condition that an appropriate person or body not party to the particular action, suit or proceeding shall not have determined that the executive is not permitted to be indemnified under applicable law. The indemnification agreements also set forth procedures that apply in the event an executive requests indemnification or an expense advance.
Summary Compensation Table
The table below provides summary information concerning all compensation awarded to, earned by or paid to our named executive officers during our 2020 and 2019 fiscal years.
|
Name and Principal Position |
Year |
Salary |
Bonus(1) |
Option Awards(2) |
Non- Equity Incentive Plan Compen- sation(3) |
All Other Compen- sation(4) |
Total |
|||||||||||||||||||
|
Rick Pauls(5) |
2020 |
$ | 455,013 | $ | — | $ | 190,624 | $ | 217,716 | $ | 14,850 | $ | 878,203 | |||||||||||||
|
President and Chief Executive Officer |
2019 |
420,000 | — | 890,736 | 178,000 | 14,650 | 733,707 | |||||||||||||||||||
|
Scott Kellen |
2020 |
276,075 | — | 119,140 | 79,238 | 14,850 | 489,303 | |||||||||||||||||||
|
Chief Financial Officer and Secretary |
2019 |
262,500 | — | 336,557 | 64,800 | 13,950 | 677,807 | |||||||||||||||||||
|
Harry Alcorn, Jr., Pharm.D. |
2020 |
291,413 | — | 119,140 | 83,662 | 14,850 | 509,065 | |||||||||||||||||||
|
Chief Medical Officer |
2019 |
273,750 | — | 421,750 | 68,400 | 14,400 | 778,300 | |||||||||||||||||||
|
(1) |
We generally do not pay discretionary bonuses. |
|
(2) |
Amounts reflect the full grant-date fair value of stock options granted during the applicable year computed in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718, rather than the amounts paid to or realized by the named individual. The grant date fair value is determined based on our Black-Scholes option pricing model. The table below sets forth the specific assumptions used in the valuation of each such option award: |
|
Grant Date |
Grant Date Fair Value Per Share |
Risk Free Interest Rate |
Expected Life (years) |
Expected Volatility |
Expected Dividend Yield |
||||||||||||
|
06/01/2020 |
$ | 3.40 | 0.32 | % |
5.1 |
97.99 | % | — | |||||||||
|
06/24/2019 |
3.37 | 1.75 | % |
5.1 |
96.3 | % | — | ||||||||||
There can be no assurance that unvested awards will vest (and, absent vesting and exercise, no value will be realized by the executive for the award).
|
(3) |
Amounts reported represent awards earned for that year under our annual short-term incentive plan but paid during the following year. See “—Executive Compensation Overview—Annual Short-Term Incentive Compensation.” |
|
(4) |
The amounts shown in the “All Other Compensation” column for fiscal 2020 include the following with respect to each named executive officer: |
|
Name |
401(k) Match |
Health Savings Account Contribution |
Total |
|||||||||
|
Rick Pauls |
$ | 11,400 | $ | 3,450 | $ | 14,850 | ||||||
|
Scott Kellen |
11,400 | 3,450 | 14,850 | |||||||||
|
Harry Alcorn, Jr., Pharm.D. |
11,400 | 3,450 | 14,850 | |||||||||
|
(5) |
Mr. Pauls is also a director of DiaMedica and did not receive any compensation related to his role as a director. |
Outstanding Equity Awards at Fiscal Year-End
The following table presents for each named executive officer information regarding outstanding equity awards held as of December 31, 2020. All of our named executive officers held stock options as of December 31, 2020 and one of our named executive officers held deferred share units.
|
Option Awards(1) |
Stock Awards |
|||||||||||||||||||
|
Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price |
Option Expiration Date(2) |
Number of Shares or Units of Stock That Have Not Vested(3) (#) |
Market Value of Shares or Units of Stock That Have Not Vested(4) ($) |
||||||||||||||
|
Rick Pauls |
||||||||||||||||||||
|
Stock Options |
10,000 | — | (CAD$) | 23.00 |
10/06/2021 |
|||||||||||||||
| 10,000 | — | (CAD$) | 34.00 |
02/15/2022 |
||||||||||||||||
| 10,000 | — | (CAD$) | 21.40 |
06/25/2023 |
||||||||||||||||
| 67,500 | — | (CAD$) | 3.00 |
12/01/2025 |
||||||||||||||||
| 42,500 | — | (CAD$) | 5.20 |
11/28/2026 |
||||||||||||||||
| 42,500 | — | (CAD$) | 6.40 |
06/19/2027 |
||||||||||||||||
| 27,917 | 5,583 | (CAD$) | 11.20 |
04/17/2028 |
||||||||||||||||
| 198,000 | 66,000 | (US$) | 4.60 | 06/23/2029 | ||||||||||||||||
| 9,333 | 46,667 | (US$) | 4.64 | 05/31/2030 | ||||||||||||||||
|
DSUs |
1,749 | (US$) |
17,735 |
|||||||||||||||||
|
Scott Kellen |
||||||||||||||||||||
|
Stock Options |
48,175 | 8,375 | (CAD$) | 11.20 |
04/17/2028 |
|||||||||||||||
| 74,183 | 24,937 | (US$) | 4.60 | 06/23/2029 | ||||||||||||||||
| 5,833 | 29,167 | (US$) | 4.64 | 05/31/2030 | ||||||||||||||||
|
Harry Alcorn, Pharm.D. |
||||||||||||||||||||
|
Stock Options |
18,750 | 6,250 | (CAD$) | 10.40 |
08/15/2028 |
|||||||||||||||
| 93,750 | 31,250 | (US$) | 4.60 | 06/23/2029 | ||||||||||||||||
| 5,833 | 29,167 | (US$) | 4.64 | 05/31/2030 | ||||||||||||||||
|
(1) |
All stock options vest in 12 equal quarterly installments over three years, except the stock options granted on June 24, 2019 which vest in eight equal quarterly installments over two years. The vesting of the stock options may be accelerated under certain circumstances, including if the recipient’s employment or service relationship with our company is involuntarily terminated. |
|
(2) |
All stock options have a 10-year term, but may terminate earlier if the recipient’s employment or service relationship with our company terminates. |
|
(3) |
All DSU awards are settled after the holder’s employment or service relationship with our company terminates. |
|
(4) |
The market value of DSU awards that have not been settled as of December 31, 2020 is based on the closing sale price of our common shares as reported by The Nasdaq Capital Market on December 31, 2020 ($10.14). |
Employee Benefit and Stock Plans
2019 Omnibus Incentive Plan
The DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan was adopted by the Board of Directors on March 14, 2019 and approved by our shareholders on May 22, 2019.
Shares Available. Subject to adjustment (as described below), the maximum number of our common shares authorized for issuance under the 2019 Plan is 2,000,000 shares. No more than 2,000,000 shares may be granted as incentive stock options and no more than 100,000 shares may be granted to any non-employee director in any one year (other than shares received in lieu of any annual cash retainer or meeting fees).
Eligible Participants. Awards may be granted to employees, non-employee directors and consultants of DiaMedica or any of our subsidiaries. A “consultant” for purposes of the 2019 Plan is one who renders services to DiaMedica or its subsidiaries that are not in connection with the offer and sale of our securities in a capital raising transaction and do not directly or indirectly promote or maintain a market for our securities.
Awards Available. The 2019 Plan permits us to grant non-statutory and incentive stock options, stock appreciation rights, restricted stock awards, restricted stock units, deferred stock units, performance awards, non-employee director awards, and other stock based awards. Awards may be granted either alone or in addition to or in tandem with any other type of award.
Transferability. Except pursuant to a testamentary will or the laws of descent and distribution or as otherwise expressly permitted by the 2019 Plan, no right or interest of any participant in an award prior to the exercise (in the case of options or stock appreciation rights) or vesting, issuance or settlement of such award will be assignable or transferable, or subjected to any lien, during the lifetime of the participant, either voluntarily or involuntarily, directly or indirectly, by operation of law or otherwise.
Termination of Employment or Other Service. The 2019 Plan provides for certain default rules in the event of a termination of a participant’s employment or other service. These default rules may be modified in an award agreement or an individual agreement between DiaMedica and a participant. If a participant’s employment or other service with DiaMedica is terminated for cause, then all outstanding awards held by such participant will be terminated and forfeited. In the event a participant’s employment or other service with DiaMedica is terminated by reason of death, disability, or retirement, then:
|
● |
All outstanding stock options (excluding non-employee director options in the case of retirement) and stock appreciation rights held by the participant will, to the extent exercisable, remain exercisable for a period of one year after such termination, but not later than the date the stock options or stock appreciation rights would otherwise expire; |
|
● |
All outstanding stock options and stock appreciation rights that are not exercisable and all outstanding restricted stock will be terminated and forfeited; and |
|
● |
All outstanding unvested restricted stock units, performance awards, and other stock-based awards held by the participant will terminate and be forfeited. However, with respect to any awards that vest based on the achievement of performance goals, if a participant’s employment or other service with DiaMedica or any subsidiary is terminated prior to the end of the performance period of such award, but after the conclusion of a portion of the performance period (but in no event less than one year), the Board of Directors may, in its sole discretion, cause shares to be delivered or payment made with respect to the participant’s award, but only if otherwise earned for the entire performance period and only with respect to the portion of the applicable performance period completed at the date of such event, with proration based on the number of months or years that the participant was employed or performed services during the performance period. |
In the event a participant’s employment or other service with DiaMedica is terminated by reason other than for cause, death, disability, or retirement, then:
|
● |
All outstanding stock options (including non-employee director options) and stock appreciation rights held by the participant that then are exercisable will remain exercisable for three months after the date of such termination, but will not be exercisable later than the date the stock options or stock appreciation rights would otherwise expire; |
|
● |
All outstanding restricted stock will be terminated and forfeited; and |
|
● |
All outstanding unvested restricted stock units, performance awards and other stock-based awards will be terminated and forfeited. However, with respect to any awards that vest based on the achievement of performance goals, if a participant’s employment or other service with DiaMedica or any subsidiary is terminated prior to the end of the performance period of such award, but after the conclusion of a portion of the performance period (but in no event less than one year), the Board of Directors may, in its sole discretion, cause shares to be delivered or payment made with respect to the participant’s award, but only if otherwise earned for the entire performance period and only with respect to the portion of the applicable performance period completed at the date of such event, with proration based on the number of months or years that the participant was employed or performed services during the performance period. |
Adjustments. In the event of any reorganization, merger, consolidation, recapitalization, liquidation, reclassification, stock dividend, stock split, combination of shares, rights offering, divestiture, or extraordinary dividend (including a spin off) or other similar change in the corporate structure or our common shares, the Board of Directors will make the appropriate adjustment or substitution in order to prevent dilution or enlargement of the rights of participants. These adjustments or substitutions may be to the number and kind of securities and property that may be available for issuance under the 2019 Plan. In order to prevent dilution or enlargement of the rights of participants, the Board of Directors may also adjust the number, kind and exercise price of securities or other property subject to outstanding awards.
Term, Termination and Amendment. Unless sooner terminated by the Board of Directors, the 2019 Plan will terminate at midnight on May 21, 2029. No award will be granted after termination of the 2019 Plan, but awards outstanding upon termination of the 2019 Plan will remain outstanding in accordance with their applicable terms and conditions and the terms and conditions of the 2019 Plan.
Subject to certain exceptions as set forth in the 2019 Plan, the Board of Directors has the authority to terminate and the Board of Directors has the authority to amend the 2019 Plan or any outstanding award agreement at any time and from time to time. No termination or amendment of the 2019 Plan or an award agreement shall adversely affect in any material way any award previously granted under the 2019 Plan without the written consent of the participant holding such award.
Prior Stock Option Plan
The DiaMedica Therapeutics Inc. Amended and Restated Stock Option Plan (Option Plan) was adopted by the Board of Directors on September 30, 2018 and by our shareholders on November 6, 2018. The Option Plan was terminated with respect to future grants upon the approval by the shareholders of the 2019 Plan. Options outstanding under the Option Plan remain outstanding in accordance with their applicable terms and conditions and the terms and conditions of the Option Plan.
Subject to the discretion of the Board of Directors, where a person ceases to be an eligible participant under the Option Plan, other than by reason of death or in the event of termination for cause, options granted to participants will cease to be exercisable on the earlier of the expiry date and 90 days after the date of termination. Subject to the discretion of the Board of Directors, if a participant is terminated for cause, all options received will terminate and cease to be exercisable upon such termination.
In the event of any change in our outstanding common shares by reason of any stock dividend, split, recapitalization, reclassification, amalgamation, merger, consolidation, combination or exchange of shares or distribution of rights to holders of shares or any other form of corporate reorganization whatsoever, an equitable adjustment will be made to the share limits in the Option Plan and any options then outstanding and the exercise price in respect of such options.
Prior Deferred Share Unit Plan
The DiaMedica Therapeutics Inc. Deferred Share Unit Plan (DSU Plan) was adopted by the Board of Directors on August 25, 2011 and by our shareholders on September 22, 2011. The DSU Plan was terminated with respect to future grants upon the approval by the shareholders of the 2019 Plan. DSU awards outstanding under the DSU Plan remain outstanding in accordance with their applicable terms and conditions and the terms and conditions of the DSU Plan. All DSU awards held by a recipient settle and the shares underlying such awards become issuable only after the termination of the recipient’s employment or other service with DiaMedica.
Anti-Hedging and Pledging Policy
DiaMedica has determined that there is a heightened legal risk and/or the appearance of improper or inappropriate conduct if officers, directors and employees engage in certain types of transactions in DiaMedica’s securities that hedge or offset, or are designed to hedge or offset, any decrease in the market value of DiaMedica’s equity securities. Therefore, DiaMedica’s Insider Trading Policy provides that officers, directors and employees must comply with the following policies with respect to certain transactions in DiaMedica’s securities:
|
● |
Short Sales. Short sales of DiaMedica’s securities evidence an expectation on the part of the seller that the securities will decline in value, and therefore signal to the market that the seller has no confidence in DiaMedica or its short-term prospects. In addition, short sales may reduce the seller’s incentive to improve the Company’s performance. For these reasons, short sales of DiaMedica’s securities are prohibited. |
|
● |
Publicly Traded Options. A transaction in options is, in effect, a bet on the short-term movement of DiaMedica’s common shares and therefore creates the appearance that an officer, director or employee is trading based on inside information. Transactions in options also may focus an officer’s, director’s or employee’s attention on short-term performance at the expense of the Company’s long-term objectives. Accordingly, transactions in puts, calls or other derivative securities involving DiaMedica’s equity securities, on an exchange or in any other organized market, are prohibited. |
|
● |
Hedging Transactions. Certain forms of hedging or monetization transactions, such as zero-cost collars and forward sale contracts, allow an officer, director or employee to lock in much of the value of his or her stock holdings, often in exchange for all or part of the potential for upside appreciation in the stock. These transactions allow the officer, director or employee to continue to own the covered securities, but without the full risks and rewards of ownership. When that occurs, the officer, director or employee may no longer have the same objectives as DiaMedica’s other shareholders. Therefore, such transactions involving DiaMedica’s equity securities are prohibited. |
|
● |
Purchases of the Company’s Securities on Margin; Pledging the Company’s Securities to Secure Margin or Other Loans. Purchasing on margin means borrowing from a brokerage firm, bank or other entity in order to purchase DiaMedica’s securities (other than in connection with a cashless exercise of stock options through a broker under the Company’s equity plans). Margin purchases of DiaMedica’s securities are prohibited. Pledging DiaMedica’s securities as collateral to secure loans is also prohibited. This prohibition means, among other things, that directors, officers and employees cannot hold DiaMedica’s securities in a “margin account.” |
Director Compensation
Non-Employee Director Compensation Program
Overview. Our non-employee directors currently consist of Michael Giuffre, M.D., James Parsons, and Richard Pilnik.
We use a combination of cash and long-term equity-based incentive compensation in the form of stock option grants to attract and retain qualified candidates to serve on the Board of Directors. In setting non-employee director compensation, we follow the process and procedures described under “Item 10. Directors, Executive Officers and Corporate Governance –Compensation Committee—Processes and Procedures for the Determination of Director Compensation.”
In April 2020, we reviewed our non-employee director compensation program in light of a benchmarking analysis performed by our independent compensation consultant, 21-Group. The peer group used for this benchmarking analysis was the same peer group used for the executive compensation analysis. As a result of this review, we increased the cash retainers for our Board committee chairs and instituted new cash retainers for our Board committee members to bring their compensation closer to our target market positioning.
Cash Retainers. The following table sets forth the annual cash retainers paid to our non-employee directors during fiscal 2020:
|
Description |
Fiscal 2020 Annual Cash Retainer Prior to April 1, 2020 |
Fiscal 2020 Annual Cash Retainer Effective April 1, 2020 |
||||||
|
Board Member |
$ | 40,000 | $ | 40,000 | ||||
|
Chairman of the Board |
20,000 | 20,000 | ||||||
|
Audit Committee Chair |
8,000 | 15,000 | ||||||
|
Audit Committee Member |
0 | 7,500 | ||||||
|
Compensation Committee Chair |
4,000 | 10,000 | ||||||
|
Compensation Committee Member |
0 | 5,000 | ||||||
|
Nominating and Corporate Governance Committee Chair |
4,000 | 7,500 | ||||||
|
Nominating and Corporate Governance Committee Member |
0 | 3,750 | ||||||
Stock Options. During 2020, each of our non-employee directors received a stock option with a value of $45,000 and our Chairman of the Board received an additional option with a value of $20,000. Accordingly, on June 1, 2020, each of our non-employee directors received an option to purchase 13,306 common shares at an exercise price equal to $4.64 per share and our Chairman of the Board received an additional option to purchase 5,914 common shares at an exercise price equal to $4.64 per share. These options expire on May 31, 2030 and vest in four nearly equal quarterly installments over one year.
Deferred Stock Units or Restricted Stock Units. We provide our non-employee directors the opportunity to elect to receive Deferred Stock Units (DSUs) or Restricted Stock Units (RSUs) in lieu of up to 100% of their annual cash retainers payable for services to be rendered as a non-employee director, chairman and chair or member of any board committee. Effective as of the first business day of each year (or June 1, 2020 in the case of DSU or RSU awards applicable to the 2020 transition period or the first date as a director in the case of new directors), each of our non-employee directors who elected to receive DSUs or RSUs in lieu of all or a portion of such director’s annual cash retainers, were granted DSU or RSU awards under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan (2019 Plan) covering that number of shares as determined based on the following formula (rounding down to the nearest whole share):
|
● |
the aggregate dollar amount of the elected portion of the annual cash retainers that otherwise would have been payable to the non-employee director for services to be rendered as a non-employee director, Chairman of the Board and Chair or member of any Board committee during the year (or transition or other period, if applicable) based on such director’s Board committee memberships and Chair positions as of the date of grant, divided by |
|
● |
the 10-trading day average closing sale price of our common shares, as reported by the Nasdaq Capital Market, and as determined on the third (3rd) business day prior to the anticipated grant date of the award. |
Such DSU and RSU awards vest in four as nearly equal as possible quarterly installments, on March 31, June 30, September 30 and December 31, in each case so long as the non-employee director is a director of the Company as of such date. DSU awards are settled following a separation from service by such director and RSU awards are settled immediately upon vesting or, if earlier, the death of the director.
If a non-employee director who elected to receive an DSU or RSU award in lieu of all or a portion of such director’s annual cash retainers is no longer a director of the Company before such director’s interest in all of the shares underlying the DSU or RSU award have vested, the director will forfeit his or her rights to receive all of such unvested shares on the day his or her status as a director of the Company terminates. However, shares underlying the DSU or RSU award corresponding to the elected cash retainers for such quarter in which the director’s status changed will vest ratably for such quarter based on the number of days of service as a director of the Company during such quarter.
If a non-employee director of the Company who elected to receive an DSU or RSU award in lieu of his or her annual cash retainers becomes entitled to receive an increased or additional annual cash retainer during the year, the director will receive such increased or additional annual cash retainer in cash until the director makes his or her election for the following year. Conversely, if a non-employee director of the Company who elected to receive an DSU or RSU award in lieu of such director’s annual cash retainers experiences a change in committee membership or Chair positions prior to year end, such that the aggregate amount of annual cash retainers for the year to which the director is entitled is less than the aggregate amount used to calculate the director’s most recent DSU or RSU award, the director will forfeit effective as of such change his or her rights to receive the corresponding portion of the shares underlying such DSU or RSU award; provided, however, that in the event the director elected to receive only a portion of his or her cash retainers in the form of an DSU or RSU award, the amount of cash retainers to be received will be reduced first. In addition, in the event shares underlying the DSU or RSU award are forfeited, the vesting of the DSU or RSU award will be revised accordingly as of the date of such change.
Director Compensation Table
The table below provides summary information concerning the compensation of each individual who served as a director of our company during the fiscal year ended December 31, 2020, other than Rick Pauls, our President and Chief Executive Officer, who was not compensated separately for serving on the Board of Directors during fiscal 2020. His compensation during fiscal 2020 for serving as an executive officer of our company is set forth under “—Executive Compensation Overview” and “—Summary Compensation Table.”
|
Name |
Fees Earned or Paid in Cash(1) |
Option Awards(2)(3) |
Stock Awards(4) |
All Other Compensation |
Total |
|||||||||||||||
|
Michael Giuffre, M.D. |
$ | 56,938 | $ | 45,294 | $ | 389 | $ | — | $ | 102,621 | ||||||||||
|
James Parsons |
59,813 | 45,294 | 405 | — | 105,512 | |||||||||||||||
|
Richard Pilnik |
76,000 | 65,425 | 513 | — | 141,938 | |||||||||||||||
|
Zhenyu Xiao, Ph.D.(5) |
16,904 | — | — | — | 16,904 | |||||||||||||||
|
(1) |
The following directors elected to receive DSUs for part of their retainers: Giuffre ($35,729 was paid in the form of 7,784 DSUs); Parsons ($37,188 was paid in the form of 8,102 DSUs); and Pilnik ($46,667 was paid in the form of 10,168 DSUs). |
|
(2) |
Amounts reflect the grant date fair value for option awards granted to each non-employee director computed in accordance with FASB ASC Topic 718. |
|
(3) |
The following directors held the following option awards as of December 31, 2020: Giuffre (57,506 options); Parsons (51,256 options); and Pilnik (96,670 options). |
|
(4) |
Represents the difference between the grant date fair value of the DSUs received by the director, using the grant date fair value of $4.64 per share, and the dollar amount of retainers used to calculate the number of DSUs, using an average stock price of $4.59 per share. |
|
(5) |
Zhenyu Xiao, Ph.D. served as a director until the 2020 Annual General Meeting of Shareholders held on June 2, 2020. |
Indemnification
Our Articles provide that subject to British Columbia’s Business Corporations Act (BCBCA), we will indemnify a director or a former director (each an “eligible party”) and his or her heirs and legal representatives, against all eligible penalties to which such person is liable. The Company must pay the expenses actually and reasonably incurred by such person in respect of any eligible proceeding either as they are incurred in advance of the final disposition of the proceeding or after the final disposition of a proceeding. Our Articles define an “eligible penalty” as a judgment, penalty or fine awarded or imposed in, or an amount paid in settlement of, an eligible proceeding. Our Articles define an “eligible proceeding” as a legal proceeding or investigative action, whether current, threatened, pending or completed, in which an eligible party or any of the heirs and legal personal representatives of the eligible party, by reason of the eligible party being or having been a director of the Company: (i) is or may be joined as a party; or (ii) is or may be liable for or in respect of a judgment, penalty or fine in, or expenses related to, the proceeding.
We entered into indemnification agreements with all of our directors, which are nearly identical to the indemnification agreements with our executive officers as described under “—Executive Compensation Overview—Indemnification Agreements.”
At present, there is no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Insofar as indemnification for liabilities arising under the United States Securities Act of 1933, as amended (Securities Act) may be permitted to directors, executive officers or persons controlling us, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Security Ownership of Significant Beneficial Owners
The table below sets forth information as to entities that have reported to the Securities and Exchange Commission (SEC) or have otherwise advised us that they are a beneficial owner, as defined by the SEC’s rules and regulations, of more than five percent of our common shares.
|
Title of Class |
Name and Address of Beneficial Owner |
Amount and Nature of Beneficial Ownership |
Percent of Class(1) |
|||||||
|
Common Shares |
Stonepine Capital Management, LLC 919 NW Bond Street, Suite 204 Bend, OR 97703-2767 |
950,000 | (2) | 5.1 | % | |||||
|
Nantahala Capital Management |
||||||||||
|
(1) |
Percent of class is based on 18,786,157 shares outstanding as of April 26, 2021. |
|
(2) |
Based solely on information contained in a Schedule 13G of Stonepine Capital Management, LLC filed with the SEC on February 12, 2021, reflecting beneficial ownership as of December 31, 2020. Stonepine Capital Management, LLC (GP) is the record owner of 950,000 shares. The GP is the general partner and investment advisor of investment funds, including Stonepine Capital, L.P. (LP), Jon M. Plexico and Timothy P. Lynch are the control persons of the GP. The GP, LP, Mr. Plexico and Mr. Lynch filed their Schedule 13G jointly, but not as members of a group, and each disclaims membership in a group. Each of the GP, LP, Mr. Plexico and Mr. Lynch disclaim beneficial ownership of these shares, except to the extent of that person’s pecuniary interest therein. |
Security Ownership of Management
The table below sets forth information known to us regarding the beneficial ownership of our common shares as of April 26, 2021,by:
|
● |
each of our current directors; |
|
● |
each of the individuals named in the Summary Compensation Table under “Item 11. Executive Compensation – Summary Compensation Table”; and |
|
● |
all of our current directors and executive officers as a group. |
To our knowledge, each person named in the table has sole voting and investment power with respect to all of the securities shown as beneficially owned by such person, as determined by the rules of the SEC, except as otherwise set forth in the notes to the table and subject to community property laws, where applicable. The SEC has defined “beneficial” ownership of a security to mean the possession, directly or indirectly, of voting power and/or investment power. A shareholder is also deemed to be, as of any date, the beneficial owner of all securities that such shareholder has the right to acquire within 60 days after that date through (i) the exercise of any option, warrant or right; (ii) the conversion of a security; (iii) the power to revoke a trust, discretionary account or similar arrangement; or (iv) the automatic termination of a trust, discretionary account or similar arrangement. However, such unissued shares of common shares are not deemed to be outstanding for calculating the percentage of common shares owned by any other person.
Unless otherwise indicated below, the address for each beneficial owner listed is c/o DiaMedica Therapeutics Inc., Two Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447.
|
Title of Class |
Name of Beneficial Owner |
Amount and Nature of Beneficial Ownership(1) |
Percent of Class(2) |
|||||||
|
Common Shares |
Richard Pilnik |
149,760 | * | |||||||
|
Common Shares |
Michael Giuffre, M.D. |
234,585 | (3) | 1.2 | % | |||||
|
Common Shares |
James Parsons |
53,506 | * | |||||||
|
Common Shares |
Rick Pauls |
527,722 | 2.7 | % | ||||||
|
Common Shares |
Scott Kellen |
173,957 | * | |||||||
|
Common Shares |
Harry Alcorn, Jr., Pharm.D. |
169,927 | * | |||||||
|
Common Shares |
All current directors and executive officers as a group (7 persons) |
1,340,290 | 6.8 | % | ||||||
|
* |
Represents beneficial ownership of less than one percent. |
|
(1) |
Includes for the persons listed below the following shares subject to options held by such persons that are currently exercisable or become exercisable within 60 days of April 26, 2021: |
|
Name |
Shares Underlying Stock Options |
|||
|
Directors |
||||
|
Richard Pilnik |
96,670 | |||
|
Michael Giuffre, M.D. |
57,506 | |||
|
James Parsons |
51,256 | |||
|
Rick Pauls |
498,667 | |||
|
Name Executive Officers |
||||
|
Rick Pauls |
498,667 | |||
|
Scott Kellen |
161,667 | |||
|
Harry Alcorn, Jr., Pharm.D. |
161,667 | |||
|
All current directors and executive officers as a group (7 persons) |
1,058,266 | |||
Excludes the following common shares issuable upon the settlement of deferred share unit awards, which will be settled after the holder’s employment or service relationship with DiaMedica terminates: Mr. Pilnik (27,475 shares); Mr. Pauls (1,749 shares); Dr. Giuffre (19,371 shares); and Mr. Parsons (19,697 shares).
|
(2) |
Percent of class is based on 18,786,157 shares outstanding as of April 26, 2021. |
|
(3) |
Includes: (i) 5,165 shares held by 424822 Alberta Ltd, over which Dr. Giuffre has sole voting and dispositive power , (ii) 36,498 shares Dr. Giuffre and his wife hold jointly, (iii) 54,186 shares held by Dr. Giuffre’s sons and daughters, (iv) 21,070 common shares held by Dr. Giuffre’s wife and (v) 60,160 shares held directly by Dr. Giuffre. |
Securities Authorized for Issuance under Equity Compensation Plans
The following table summarizes outstanding options and other awards under our equity compensation plans as of December 31, 2020. Our equity compensation plans as of December 31, 2020 were the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan (2019 Plan), the DiaMedica Therapeutics Inc. Stock Option Plan, Amended and Restated November 6, 2018 (Prior Plan) and the DiaMedica Therapeutics Inc. Amended and Restated Deferred Share Unit Plan (DSU Plan).
Equity Compensation Plan Information
|
(a) |
(b) |
(c) |
||||||||||
|
Plan Category |
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) |
|||||||||
|
Equity compensation plans approved by security holders |
1,436,803 | (1) | $ | 5.24 | (2) | 1,099,098 | (3) | |||||
|
Equity compensation plans not approved by security holders |
— | $ | — | — | ||||||||
|
Total |
1,436,803 | (1) | $ | 5.24 | (2) | 1,099,098 | (3) | |||||
______________________
|
(1) |
Amount includes 873,182 common shares issuable upon the exercise of stock options and 26,054 common shares issuable upon the settlement of DSU awards outstanding under the 2019 Plan, 516,384 common shares issuable upon the exercise of stock options under the Prior Plan and 21,183 common shares issuable under the DSU Plan. |
|
(2) |
Not included in the weighted-average exercise price calculation are 26,054 deferred share unit awards under the 2019 Plan and 21,183 deferred share unit awards under the DSU Plan. |
|
(3) |
Amount includes 1,099,098 shares remaining available for future issuance under the 2019 Plan. |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
Introduction
Below under “—Description of Related Party Transactions” is a description of transactions that have occurred during the past fiscal year, or any currently proposed transactions, to which we were or are a participant and in which:
|
● |
the amounts involved exceeded or will exceed the lesser of: $120,000 or one percent (1%) of the average of our total assets at year end for the last two completed fiscal years; and |
|
● |
a related person (including any director, director nominee, executive officer, holder of more than 5% of our common shares or any member of their immediate family) had or will have a direct or indirect material interest. |
Description of Related Party Transactions
Agreement with Trident Rx Consulting Services LLC
We have engaged the services of Trident Rx Consulting Services LLC, a limited liability company owned by Dr. Sydney Gilman, our Vice President of Regulatory Affairs, to perform regulatory consulting services for us. The fees we pay are based solely on the hourly fees of the consultants performing services for us. There is no markup received by Dr. Gilman. During 2020, we paid $235,143 to Trident Rx Consulting Services LLC under this arrangement. The Audit Committee reviewed the purpose of the transaction, the benefits of the transaction, the availability of other sources for comparable services, the terms of the transaction, and the terms available to unrelated third parties or employees generally and determined in good faith that the transaction is in, and not inconsistent with, the best interests of the Company.
Relationship with Hermeda Industrial Co., Limited
We and Hermeda Industrial Co., Limited (Hermeda) were parties to an investment agreement, which included terms relating to the composition of the Board of Directors. Under director nomination provisions of this agreement, Hermeda had the right to designate a representative to be nominated to the Board of Directors for so long as Hermeda beneficially owned at least 10% of our outstanding common shares on a non-diluted basis, and we agreed to use our reasonable best efforts to cause the Hermeda designee to be elected. Zhenyu Xiao, Ph.D., a former director who did not stand for re-election at our 2020 Annual General Meeting, was the designee of Hermeda under the investment agreement. Currently Hermeda beneficially owns less than five percent of our outstanding common shares.
Indemnification Agreements
We have entered into indemnification agreements with all of our directors and executive officers. The indemnification agreements provide, among other things, for indemnification, to the fullest extent permitted by law and our Articles, against any and all expenses (including attorneys’ fees) and liabilities, judgments, fines and amounts paid in settlement that are paid or incurred by the executive or on his or her behalf in connection with such action, suit or proceeding. The indemnification agreements also set forth procedures that apply in the event an executive requests indemnification or an expense advance. For more information regarding these agreements, see “Item 11. Executive Compensation – Director Compensation—Indemnification.”
DiaMedica has not identified any arrangements or agreements relating to compensation provided by a third party to DiaMedica’s directors or director nominees in connection with their candidacy or board service as required to be disclosed pursuant to Nasdaq Rule 5250(b)(3).
Policies and Procedures for Related Party Transactions
The Board of Directors has delegated to the Audit Committee, pursuant to the terms of a written policy and the formal written charter of the Audit Committee, the authority to review, approve and ratify related party transactions. If it is not feasible for the Audit Committee to take an action with respect to a proposed related party transaction, the Board of Directors or another committee, may approve or ratify it. No member of the Board of Directors or any committee may participate in any review, consideration or approval of any related party transaction with respect to which such member or any of his or her immediate family members is the related party.
Our policy defines a “related party transaction” as a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we (including any of our subsidiaries and affiliates) were, are or will be a participant and in which any related party had, has or will have a direct or indirect interest (other than solely as a result of being a director or less than 10 percent beneficial owner of another entity).
Prior to entering into or amending any related party transaction, the party involved must provide notice to our Chief Financial Officer of the facts and circumstances of the proposed transaction, including:
|
● |
the related party’s relationship to us and his or her interest in the transaction; |
|
● |
the material facts of the proposed related party transaction, including the proposed aggregate value of such transaction or, in the case of indebtedness, the amount of principal that would be involved; |
|
● |
the purpose and benefits of the proposed related party transaction with respect to us; |
|
● |
if applicable, the availability of other sources of comparable products or services; and |
|
● |
an assessment of whether the proposed related party transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
If the Chief Financial Officer determines the proposed transaction is a related party transaction in which the amount involved will or may be expected to exceed $10,000 in any calendar year, the proposed transaction will be submitted to the Audit Committee for consideration. In determining whether to approve a proposed related party transaction, the Audit Committee, or where submitted to the Chair of the Audit Committee, the Chair of the Audit Committee, will consider, among other things, the following:
|
● |
the purpose of the transaction; |
|
● |
the benefits of the transaction to us; |
|
● |
the impact on a director’s independence in the event the related party is a non-employee director, an immediate family member of a non-employee director or an entity in which a non-employee director is a partner, shareholder or executive officer; |
|
● |
the availability of other sources for comparable products or services; |
|
● |
the terms of the transaction; and |
|
● |
the terms available to unrelated third parties or to employees generally. |
Under our policy, certain related party transactions as defined under our policy will be deemed to be pre-approved by the Audit Committee and will not be subject to these procedures.
Director Independence
The Board of Directors has affirmatively determined that three of DiaMedica’s current four directors are “independent directors” under the Nasdaq Listing Rules: Richard Pilnik, Michael Giuffre, M.D. and James Parsons. In making these affirmative determinations that such individuals are “independent directors,” the Board of Directors reviewed and discussed information provided by the directors and by DiaMedica with regard to each director’s business and personal activities as they may relate to DiaMedica and our management.
|
Item 14. |
Principal Accounting Fees and Services |
Audit, Audit-Related, Tax and Other Fees
The following table presents the aggregate fees billed to us by Baker Tilly US, LLP for the fiscal years ended December 31, 2020 and December 31, 2019.
|
Aggregate Amount Billed by Baker Tilly US, LLP ($) |
||||||||
|
Fiscal 2020 |
Fiscal 2019 |
|||||||
|
Audit Fees(1) |
$ | 111,500 | $ | 106,500 | ||||
|
Audit-Related Fees(2) |
73,500 | 6,500 | ||||||
|
Tax Fees |
— | — | ||||||
|
All Other Fees |
— | — | ||||||
|
(1) |
These fees consisted of the audit of our annual consolidated financial statements for fiscal 2020 and 2019, review of quarterly consolidated financial statements and other services normally provided in connection with statutory and regulatory filings or engagements. |
|
(2) |
These fees consisted of the review of our registration statement on Form S-3 in 2020 and registration statements on Form S-8 and Form S-3 in 2019 and related services normally provided in connection with statutory and regulatory filings or engagements. |
Audit Committee Pre-Approval Policies and Procedures
All services rendered by Baker Tilly US, LLP to DiaMedica were permissible under applicable laws and regulations and all services provided to DiaMedica, other than de minimis non-audit services allowed under applicable law, were approved in advance by the Audit Committee. The Audit Committee’s formal written charter requires the Audit Committee to pre-approve all auditing services and permitted non-audit services, including fees for such services. In accordance with applicable law, the Audit Committee may delegate to one or more designated members of the Audit Committee the authority to grant the required pre-approvals, provided that the decisions of any member(s) to whom such authority is delegated to pre-approve an activity shall be presented to the full Audit Committee at its next regularly scheduled meeting. The Audit Committee has delegated to the Audit Committee Chair the authority to grant the required pre-approvals for any engagement that does not exceed $25,000.
PART IV
|
Item 15. |
Exhibits and Financial Statement Schedules |
Financial Statements
Our consolidated financial statements are included in “Part II, Item 8. Financial Statements and Supplementary Data.”
Financial Statement Schedules
All financial statement schedules are omitted because they are inapplicable since we are a smaller reporting company.
Exhibits
The exhibits being filed or furnished with this report are listed below, along with an indication as to each management contract or compensatory plan or arrangement.
A copy of any of the exhibits listed or referred to herein will be furnished at a reasonable cost to any person who is a shareholder upon receipt from any such person of a written request for any such exhibit. Such request should be sent to: Mr. Scott Kellen, Chief Financial Officer and Corporate Secretary, DiaMedica Therapeutics Inc., Two Carlson Parkway, Suite 260, Minneapolis, Minnesota 55447, Attn: Shareholder Information.
|
Item No. |
Item |
Method of Filing |
||
|
3.1 |
Notice of Articles of DiaMedica Therapeutics Inc. dated May 31, 2019 |
Incorporated by reference to Exhibit 3.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 4, 2019 (File No. 001-36291) | ||
|
3.2 |
Incorporated by reference to Exhibit 3.2 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 4, 2019 (File No. 001-36291) |
|||
|
4.1 |
Description of Securities Registered Pursuant to Section 12 of the Securities Exchange Act of 1934 |
Incorporated by reference to Exhibit 4.8 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
||
|
4.2 |
Specimen Certificate representing Voting Common Shares of DiaMedica Therapeutics Inc. |
Incorporated by reference to Exhibit 4.2 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 4, 2019 (File No. 001-36291) |
| Item No. | Item | Method of Filing | ||
|
4.3 |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on December 11, 2018 |
|||
|
4.4 |
Incorporated by reference to Exhibit 4.8 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2019 (File No. 001-36291) |
|||
|
4.5 |
Incorporated by reference to Exhibit 4.1 to DiaMedica’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2020 (File No. 001-36291) |
|||
|
10.1# |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on May 23, 2019 (File No. 001-36291) |
|||
|
10.2# |
Form of Option Award Agreement under the DiaMedica Therapeutics Inc. 2019 Omnibus Incentive Plan |
Incorporated by reference to Exhibit 10.2 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 21, 2019 (File No. 001-36291) |
||
|
10.3# |
Incorporated by reference to Exhibit 10.3 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 21, 2019 (File No. 001-36291) |
|||
|
10.4# |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2020 (File No. 001-36291) |
|||
|
10.5# |
DiaMedica Therapeutics Inc. Stock Option Plan Amended and Restated November 6, 2018 |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
||
|
10.6# |
Incorporated by reference to Exhibit 10.3 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
| Item No. | Item | Method of Filing | ||
|
10.7# |
Incorporated by reference to Exhibit 10.2 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
|||
|
10.8# |
DiaMedica Therapeutics Inc. Amended and Restated Deferred Share Unit Plan |
Incorporated by reference to Exhibit 10.4 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
||
|
10.9# |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 21, 2019 (File No. 001-36291) |
|||
|
10.10# |
Form of Indemnification Agreement between DiaMedica Therapeutics Inc. and Each Director and Officer |
Incorporated by reference to Exhibit 10.1 to DiaMedica’s Current Report on Form 8-K as filed with the Securities and Exchange Commission on June 4, 2019 (File No. 001-36291) |
||
|
10.11# |
Employment Agreement effective as of September 12, 2018 between DiaMedica USA, Inc. and Rick Pauls |
Incorporated by reference to Exhibit 10.6 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
||
|
10.12# |
Employment Agreement effective as of September 12, 2018 between DiaMedica USA, Inc. and Scott Kellen |
Incorporated by reference to Exhibit 10.7 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2018 (File No. 001-36291) |
||
|
10.13# |
Incorporated by reference to Exhibit 10.9 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2018 (File No. 001-36291) |
|||
|
10.14 |
Incorporated by reference to Exhibit 10.8 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
| Item No. | Item | Method of Filing | ||
|
10.15 |
Incorporated by reference to Exhibit 10.9 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
|||
|
10.16 |
First Amendment to Lease dated May 3, 2017 between One Two Holding LLC and DiaMedica USA Inc. |
Incorporated by reference to Exhibit 10.10 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
||
|
10.17 |
Second Amendment to Lease dated September 5, 2017 between One Two Holding LLC and DiaMedica USA Inc. |
Incorporated by reference to Exhibit 10.11 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
||
|
10.18(1) |
Incorporated by reference to Exhibit 10.12 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
|||
|
10.19 |
Incorporated by reference to Exhibit 10.13 to DiaMedica’s Registration Statement on Form S-1 as filed with the Securities and Exchange Commission on November 9, 2018 (File No. 333-228313) |
|||
|
10.20 |
Incorporated by reference to Exhibit 10.19 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2019 (File No. 001-36291) |
|||
|
21.1 |
Incorporated by reference to Exhibit 21.1 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2019 (File No. 001-36291) |
|||
|
23.1 |
Incorporated by reference to Exhibit 23.1 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
| Item No. | Item | Method of Filing | ||
|
31.1 |
Incorporated by reference to Exhibit 31.1 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
|||
|
31.2 |
Incorporated by reference to Exhibit 31.2 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
|||
|
31.3 |
Filed herewith |
|||
|
31.4 |
Filed herewith |
|||
|
32.1 |
Incorporated by reference to Exhibit 32.1 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
|||
|
32.2 |
Incorporated by reference to Exhibit 32.2 to DiaMedica’s Annual Report on Form 10-K for the year ended December 31, 2020 (File No. 001-36291) |
|||
|
101 |
The following materials from DiaMedica Therapeutics Inc.’s Annual Report on Form 10-K for the year ended December 31, 2020, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Income (Loss), (iv) the Consolidated Statements of Equity, (v) the Consolidated Statements of Cash Flows, and (vi) Notes to Consolidated Financial Statements |
Previously filed |
__________________________
|
# |
Indicates a management contract or compensatory plan or arrangement. |
|
(1) |
Portions of this exhibit have been redacted and are subject to an order granting confidential treatment under Rule 406 of the United States Securities Act of 1933, as amended (File No. 333-228313, CF #36833). The redacted material was filed separately with the Securities and Exchange Commission. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Amendment No. 1 to its Annual Report on Form 10-K/A to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
DIAMEDICA THERAPEUTICS INC. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Date: April 29, 2021 |
By: |
/s/ Rick Pauls |
|
|
|
|
Rick Pauls |
|
|
|
|
President and Chief Executive Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this Amendment No. 1 to its Annual Report on Form 10-K/A has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Name |
Title |
Date |
||
| /s/ Rick Pauls | President, Chief Executive Officer and Director | April 29, 2021 | ||
| Rick Pauls | (principal executive officer) | |||
| /s/ Scott Kellen | Chief Financial Officer and Secretary | April 29, 2021 | ||
|
Scott Kellen |
(principal financial and accounting officer) |
|
||
| /s/ Richard Pilnik | Chairman of the Board | April 29, 2021 | ||
|
Richard Pilnik |
|
|
||
| /s/ Michael Giuffre, M.D. | Director | April 29, 2021 | ||
|
Michael Giuffre, M.D. |
|
|
||
| /s/ James Parsons | Director | April 29, 2021 | ||
|
James Parsons |
|
|