Controlling Blood Pressure and Improving Overall Endothelial Health
DM199 (rhKLK1) is an investigational therapy designed to improve endothelial health and support blood pressure regulation and placental perfusion in preeclampsia and fetal growth restriction. By activating natural vasodilation pathways, DM199 aims to address both maternal and fetal complications associated with vascular dysfunction, with the goal of improving outcomes for mother and baby.
Pipeline
| Indication | Compound | Route of Admin | Development Stage | |||
|---|---|---|---|---|---|---|
| Preclinical | Phase 1 | Phase 2 | Phase 2/3 | |||
| Pregnancy Complications | ||||||
| Preeclampsia | DM199 (rinvecalinase alfa) | IV/SC |
Phase 2: Part 1A Dose-Selection / Part 1B Expansion Cohort
Preclinical complete
|
Phase 1 complete
|
Phase 2 in progress
|
Phase 2/3 not started
|
| Preeclampsia | DM199 (rinvecalinase alfa) | IV/SC |
Phase 2: Parts 2 & 3 Expected Management Study
Preclinical complete
|
Phase 1 complete
|
Phase 2 in progress
|
Phase 2/3 not started
|
| Fetal Growth Restriction | DM199 (rinvecalinase alfa) | IV/SC |
Phase 2: Part 3 Fetal Growth Restriction
Preclinical complete
|
Phase 1 complete
|
Phase 2 in progress
|
Phase 2/3 not started
|
About Preeclampsia
Preeclampsia (PE) is a serious and potentially life-threatening pregnancy complication that typically occurs after 20 weeks of gestation and is a leading cause of maternal and perinatal mortality globally. PE can cause high blood pressure, proteinuria, impaired liver function, placental abruption, blood clotting and damage to other organs. There is currently no approved treatment option available to address PE. The only ‘cure’ is to deliver the baby and the placenta, often prematurely, which can lead to negative health impacts on the baby. Current standard of care involves treating symptoms in order to delay delivery.
PE occurs in two stages:
Stage 1: Placental Disease
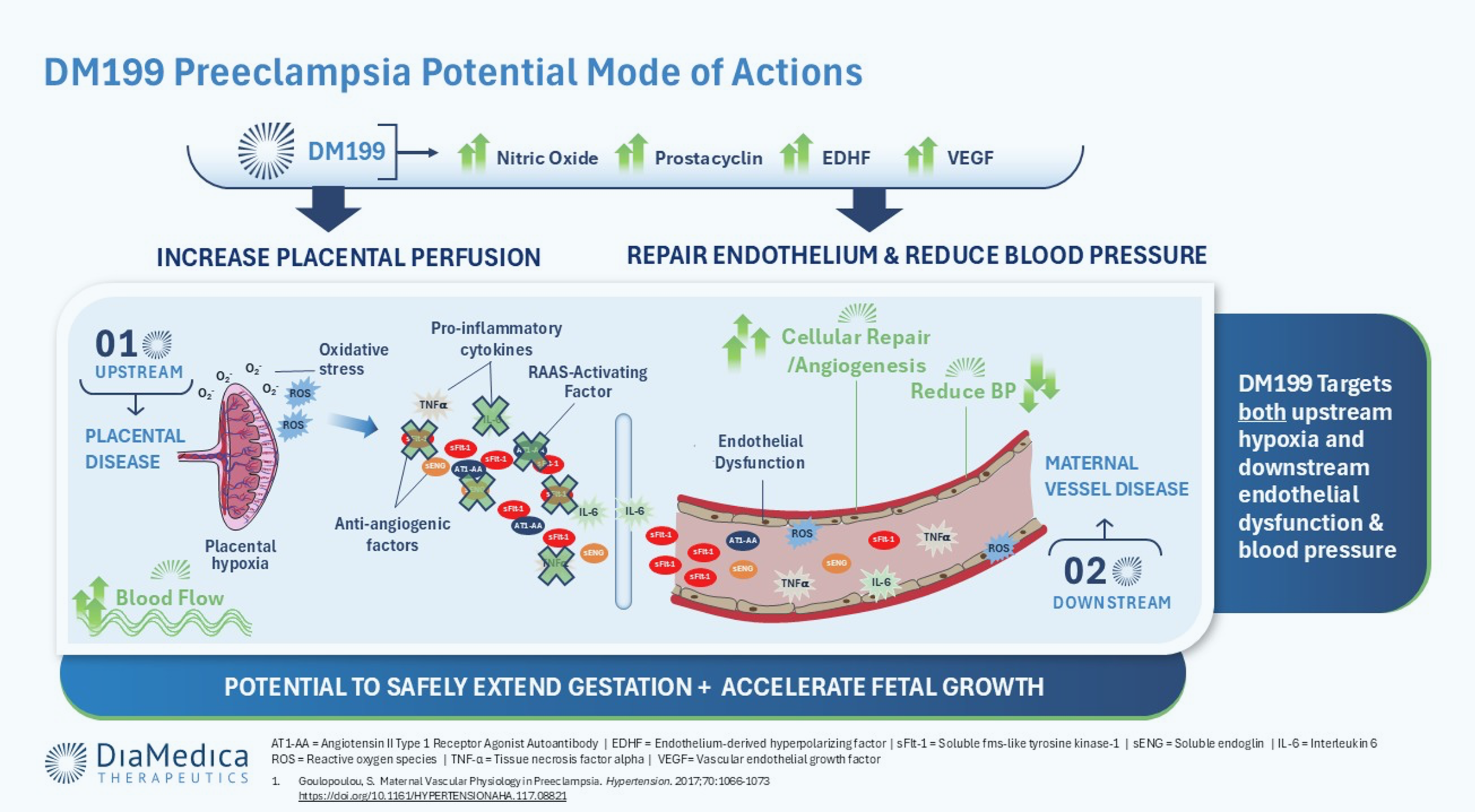
Stage 1 occurs in early pregnancy during the first trimester as the placenta fails to embed properly in the uterus. In normal pregnancies, the placenta will embed in the uterine lining and remodel the spiral arteries in the uterine wall, dilating them to promote healthy blood flow to the placenta. In PE, the spiral arteries do not remodel properly and fail to dilate, preventing adequate blood flow, oxygen, and nutrients to the placenta. This results in placental ischemia.
Stage 2: Maternal Vascular Disease and Subsequent Endothelial Dysfunction
Stage 2 of PE occurs after 20 weeks of pregnancy. The placenta, which has been chronically in need of oxygen, begins to release noxious factors that spread into maternal circulation, inflicting widespread damage to her blood vessels and leading to endothelial dysfunction. These noxious factors include damaging anti-angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), reactive oxygen species (ROS), pro-inflammatory cytokines and exosomes or cellular debris. Additionally, the production of vasodilating factors like nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarizing factor (EDHF) and vascular endothelial growth factor (VEGF) are impaired.
About Fetal Growth Restriction
Affecting up to 10% of newborns globally, fetal growth restriction (FGR) is a condition where the fetus is not growing as expected, often leading to premature birth, low birth weight, stillbirth or long-term health complications. There is no currently approved treatment option to address the underlying complications of FGR, and disease management involves monitoring the fetus, managing maternal symptoms, and oftentimes premature delivery of the baby.
FGR can often occur in patients with PE due to reduced blood flow to the placenta. Because the fetus relies heavily on the placenta for oxygen and essential nutrients, reduced blood flow to the placenta can prevent the fetus from receiving these critical elements that fuel proper growth.
A Novel Mechanism to Modify Disease
DM199 treatment is intended to counterbalance the evolution of PE by triggering the natural release of endothelial NO, PGI2, and EDHF. The activation of these signaling pathways, as was seen in the interim results of DiaMedica’s investigator sponsored PE trial, is believed to promote the vasodilation of blood vessels, and as a result, reduce maternal blood pressure, enhance placental blood flow and improve endothelial health. Additionally, clinical data demonstrate that DM199 does not cross the placental barrier, a significant safety advantage. In PE, DM199 is the only therapy in development simultaneously targeting maternal hemodynamics and fetal outcomes including FGR.
Potential Benefits of DM199 for Preeclampsia & Fetal Growth Restriction
Lower Blood Pressure • Improve Endothelial Health • Increase Maternal Organ Perfusion • Dilate Intrauterine Arteries
Clinical Studies
DiaMedica is collaborating with world leaders from Stellenbosch University, the University of Melbourne, and the University of Gothenburg who are conducting an investigator-sponsored trial. The interim Part 1a results are highly encouraging and demonstrate the best-in-class potential of DM199 as an effective therapeutic for preeclampsia. DM199 exhibited a favorable safety profile, with no placental transfer and no serious treatment-emergent adverse events reported. DM199 also produced clinically meaningful and statistically significant, dose-dependent reductions in both systolic and diastolic blood pressure across multiple timepoints. Additionally, DM199 achievedc a statistically significant improvement in uterine artery pulsatility index at the 2-hour assessment, indicating its potential for enhanced placental perfusion and disease modification.
